Human Error is the propensity for certain common mistakes by people; the making of an error as a natural result of being human. Human errors cannot be stopped by wishing they would not happen. The extent of knowledge, training and level of skill has little to do with the mistakes we make. However, most human Error is PREVENTABLE. Our systems and processes must take human factors into account and be designed to minimize or even avoid human error. In fact, human error investigations are required by regulations. The FDA has identified human error as a symptom of a deeper problem inside the Quality Management system and stated that human error investigators should have specific skills and understand human behavior and conditions that lead to human error1. Human error should be less about the person and more about why that person made the error.
To Err is Human… In Our Industry?
Errors occur in health care as well as every other very complex system that involves human beings. The message in To Err is Human was that preventing death and injury from medical errors requires dramatic, systemwide changes. Among three important strategies—preventing, recognizing, and mitigating harm from error—the first strategy (recognizing and implementing actions to prevent error) has the greatest potential effect, just as in preventive public health efforts. On average, over 60% of quality defects were attributed to human error and investigations into these root causes are generally poor and superficial2,3. There were often no sustainable CAPAs (corrective and preventive actions) defined, and the corrective action was mainly just re-training. So, what is the best way to deal with deviations and incidences which seem to be caused by human error?

The Rightness Behind Human Errors - What are the brain processes that play a role in human performance?
The brain is made up of two hemispheres, the right and the left, and they are very similar in their anatomy and their physiology. Yet, a striking number of differences in how the two hemispheres function have been described, ranging from differences in the processing of basic sensory features to differences in emotion, language, and problem solving. This disparity between the general neural similarity of the two hemispheres and the distinctiveness of their functions highlights the limits of our current understanding of the mapping between neural and functional properties (refer to Figure 1).
Human error is an inherent part of human nature and most of the time is unintentional (if not it must be assumed as falsification. It can be reduced but more investigation needs to be done on understanding the root cause of it. They are more often than not forced by the circumstances of the situation.
“Human Error occurs because we are human beings.”
- Mind’s fatigue, wander and lose concentration;
- Have varying strength and stamina;
- Numerous other human factors;
- Variability of human performance is complex;
- Hemispheres process different information types differently.
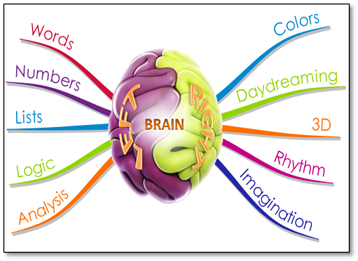
Figure 1: Human brain- Hemispheres process different information types differently.
Within the framework for Human Performance it was determined that errors occur primarily through two modes: slips and mistakes4. Slips are errors that occur during routine activities that do not require thought. These activities are so repetitive that we often think of them as things that we could do with our eyes closed. Mistakes are errors that occur during activities that require thought. They may be activities that we have some familiarity with or new activities that we have not performed before, but both require us to engage our brain and think through the situation and our actions.
The Brain Has Limitations
- Neurons fatigue within 3 to 5 minutes;
- Processes symbols more accurately and quicker than words;
- Perceives numbers, colors, and shapes as symbols;
- Cannot see UV light and have limited color memory ability;
- And there are some things your brain just cannot handle5,6!!
Holistic, Integrated Approach - Finding the Root Cause
Simply “cataloguing” and “assigning cause” to a defect or error is not compliance. Compliance presumes systems and processes are designed to adhere to regulatory pronouncements. Selecting “human error” from a dropdown list and assigning it as root cause means that user is accountable for having thoroughly investigated the causal factors of the error or defect, identifying and determining which root causes(s) are most likely, according to the preponderance of evidence, to have been associated with the defect. This means the person selecting the root cause has actually performed five (5) Whys, fishbone diagram analysis, human factors analysis, fault tree analysis, and/or many other tools for actually determining root cause(s). There are a large number of techniques and strategies that we can use for root cause analysis. Figure 2 represents root cause analysis (fishbone diagram) to discover the root causes of problems in order to identify appropriate solutions.
A fundamental, underlying, system-related reason why an incident occurred that defines a correctable failure(s) in management systems. There is typically more than one root cause for an incident. If the root cause is corrected or removed, the incident should not occur again.
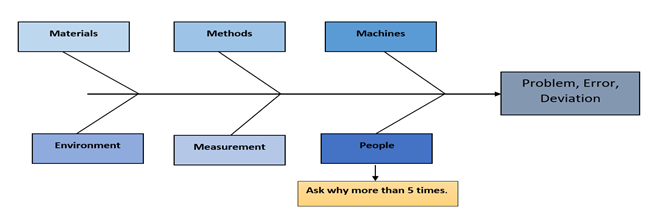
Figure 2: Root cause analysis - Fishbone diagram
Human Error Categories
Human error investigation starts when we know or suspect that it was a human action or lack of action that caused the quality defect or deviation from the process to occur (non-conformance, failure).
From Chapter 1 of the European GMPs 1.4 (xv) …Where human error is suspected or identified as the cause, this should be justified, having taken care to ensure that process, procedural or system-based errors or problems have not been overlooked, if present. In reality, most problems that appear to be caused by human error especially those that occur multiple times are actually rooted in processes or systems that when left unchanged, will keep producing the problem despite the convenient band-aids often placed over them.
When human error is identified more frequently than it should be expected to happen, it signals to investigators that problems are not being internally investigated thoroughly enough. This can quickly shift the investigator into problem-hunting mode and open your quality management system up to even greater scrutiny. It is helpful to arm with a model for analyzing what might appear to be human errors in order to determine whether actions (or inactions) were deliberate or inadvertent. The outcome can help to determine whether or not human behavior really is to blame as well as where you might expect to find a problem elsewhere (such as inadequate training, poor SOPs, etc.) if the root cause appears to be less human than you initially thought upon further analysis7.
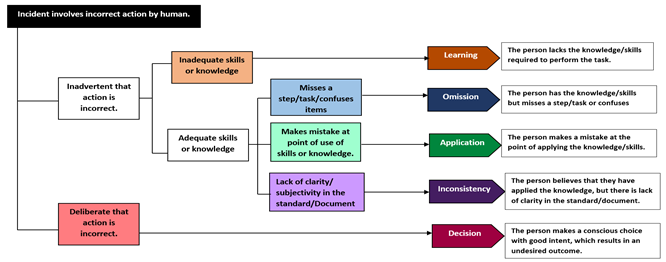
Figure 3: Human error tree diagram
Following this model (Figure 3), errors that are shown to be “inadvertent” can be considered genuinely “human,” which then fall into one of four categories: Learning, Omission, Application, or Inconsistency Mistakes4. When errors are revealed “deliberate” upon closer examination, start asking questions that cast light on how the type of work in question is actually being done. This may lead to discover the problem may be more serious and widespread, particularly in one of two areas (documentation and culture) summarized below.
Documentation
FDA-regulated manufacturing operations are followed by documentation every step of the way. If human error is found, take a hard look at your written records, and ask the questions of them listed below. The real problems may lie in your processes for creating, maintaining, and distributing the documentation that drives your quality system8.
- Are change processes long and cumbersome?
- Are your staff working with out-of-date or obsolete SOPs?
- Are your SOPs readily accessible? Can employees easily navigate to them and understand the information they need?
Culture
One of the best ways to immediately enhance quality throughout your organization is to realize most of the problems being described as human errors are something else entirely. Rather than using it as a convenient stand-in for thorough investigation, use human error as an opportunity to improve your company’s problem-solving processes. Fast closure rates of inaccurate deviations do not demonstrate efficiency, just misguided values in the problem-solving process itself.
Replace this metric with a trending reduction in total deviations over time. The size of your backlog and closure times are functions of each other and should be handled accordingly. Keep in mind that the lack of a backlog will likely lead investigators to look at your closure trends9. If your deviation backlog is reduced significantly in the weeks leading up to their arrival, they will know your methods were rushed rather than being a natural component of your QMS.
Next Steps
- Conduct an objective assessment of your internal problem-solving processes (ideally with the help of an experienced third party) and remediate accordingly;
- Replace metrics that establish problematic incentives with goals focused on long-term trending;
- Explore ways to improve problem-solving within your QMS to reduce backlogs while thoroughly investigating issues.
In Summary…
The framework of Human Performance is captured in five guiding principles:
- People are fallible, and even the best makes mistakes;
- Error-likely situations are predictable, manageable, and preventable;
- Individual behavior is influenced by organizational processes and values;
- People achieve high levels of performance based largely on the encouragement and reinforcement received from leaders, peers, and subordinates;
- Events can be avoided by an understanding of the reasons mistakes occur, and application of the lessons learned from past events.
The guiding principles of Human Performance tell us that no matter how proficient we are, we can all make mistakes; that situations in which errors occur are often predictable and therefore preventable; that we are impacted by the culture of the organization in which we perform activities; that we respond to positive reinforcement from our coworkers; and that if we tie all of this together and learn from past mistakes, we can avoid errors. Having knowledge of the performance modes and the associated error modes, we can recognize the pitfalls we face in a task and take appropriate measures to minimize the chance for error.
Interested in attending training to learn about our insights on minimising human error?
Take a look at our Behavioural GMP - Minimising Human Error course, available for both public and private delivery:
References
- https://www.pharmalex.com/human-error-some-fresh-approaches-to-consider
- https://www.pharmaceuticalonline.com/doc/human-error-is-the-leading-cause-of-gmp-deviations-or-is-it
- https://www.bioprocessonline.com/doc/human-error-deviations-why-you-should-stop-creating-most-of-them
- https://www.thefdagroup.com/blog/an-informed-guide-to-human-error-in-capa
- Book: Left Brain, Right Brain by Sally Springer, and Georg Deutsch
- Book: Hemispheric Asymmetry: What's Right and What's Left by Joseph Hellige.
- https://medium.com/the-seek-blog/human-error-and-its-impact-on-the-pharmaceutical-industry
- https://www.pharmatutor.org/articles/impact-and-management-tool-for-identification-and-reduction-of-human-errors-in-pharmaceuticals-industry
- https://incident-prevention.com/ip-articles/human-performance
