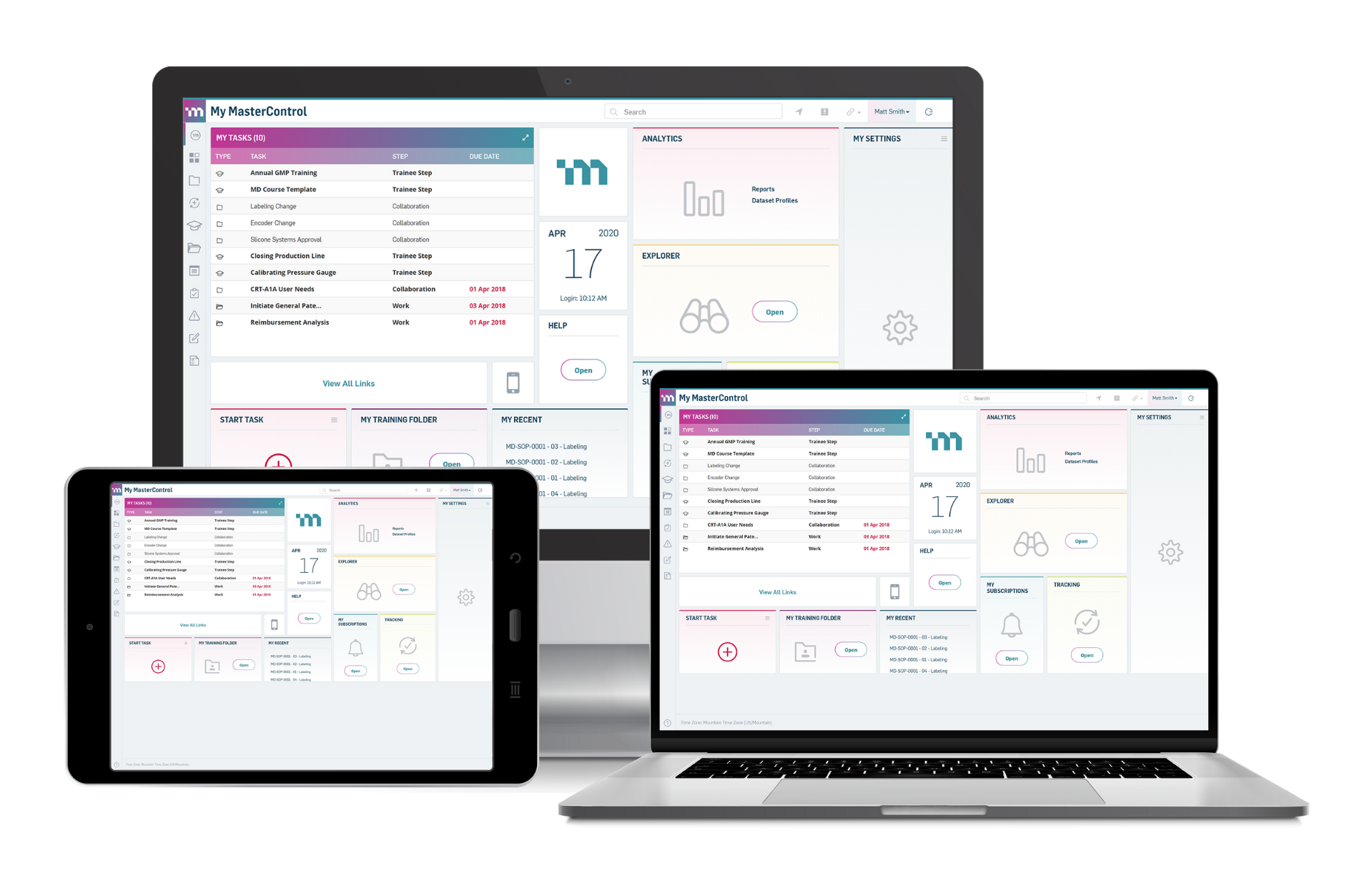
SeerPharma has over 25 years of experience implementing electronic Quality Management System (eQMS) solutions for companies operating in regulated environments. With extensive experience in the industry, we are excited to announce that we have now signed on to become a Partner for MasterControl.
SeerPharma was invited by the Stevanato Group and Lonza to present on Pharmaceutical Quality Management System (QMS) Certification at The Innovative Solutions for Pharmaceutical Packaging Roadshow in Singapore.
SeerPharma to Present on Quality Management System Certification
SeerPharma have been invited by the Stevanato Group and Lonza to present on Pharmaceutical Quality Management System Certification at The Innovative Solutions for Pharmaceutical Packaging Roadshow in Singapore.
Electronic Quality Management System (eQMS) for Pharmaceutical Company
SeerPharma’s Software Solutions team has been engaged to implement an electronic Quality Management System (eQMS) for a major multinational pharmaceutical company.
GMP Coaching Program Assists Biopharmaceutical Manufacturer
A biopharmaceutical manufacturer engaged SeerPharma to provide on-going coaching to their Quality personnel and advise on improvements to help prepare for a FDA inspection.
Pharmaceutical and Medical Device QMS Solutions
The SeerPharma team has been busy implementing electronic Quality Management Systems (eQMS) for a variety of companies ranging from pharmaceutical, nutraceutical, veterinary and medical device companies.
SeerPharma has assisted an Australian 3D Printing Medical Device company to develop an ISO 13485 compliant Quality Management System (QMS).
As a Medical Device company, you are required to investigate the cause of quality failures or production problems. Surprisingly though, “Failure to thoroughly investigate…” is a common finding from quality (management) system inspections and audits against FDA 21 CFR Part 820 and ISO 13485.
The Quality / GMP stream hosted by ISPE at this year’s ARCS congress was a great opportunity to explore the latest issues and trends. Feedback and questions raised during the Quality / GMP sessions identified three current areas of interest to many involved in this area of work. Namely the approach and use of Quality Management Systems, Validation...