Artificial Intelligence (AI) is reshaping industries worldwide, and life sciences is no exception. From drug discovery to clinical decision support, AI has the potential to transform the development, testing, and delivery of therapies. Yet, while the promise is immense, adoption remains cautious. The road ahead requires striking a balance between...
Application of ISO 14971 Risk Management to New Medical Devices
February
08,
2018
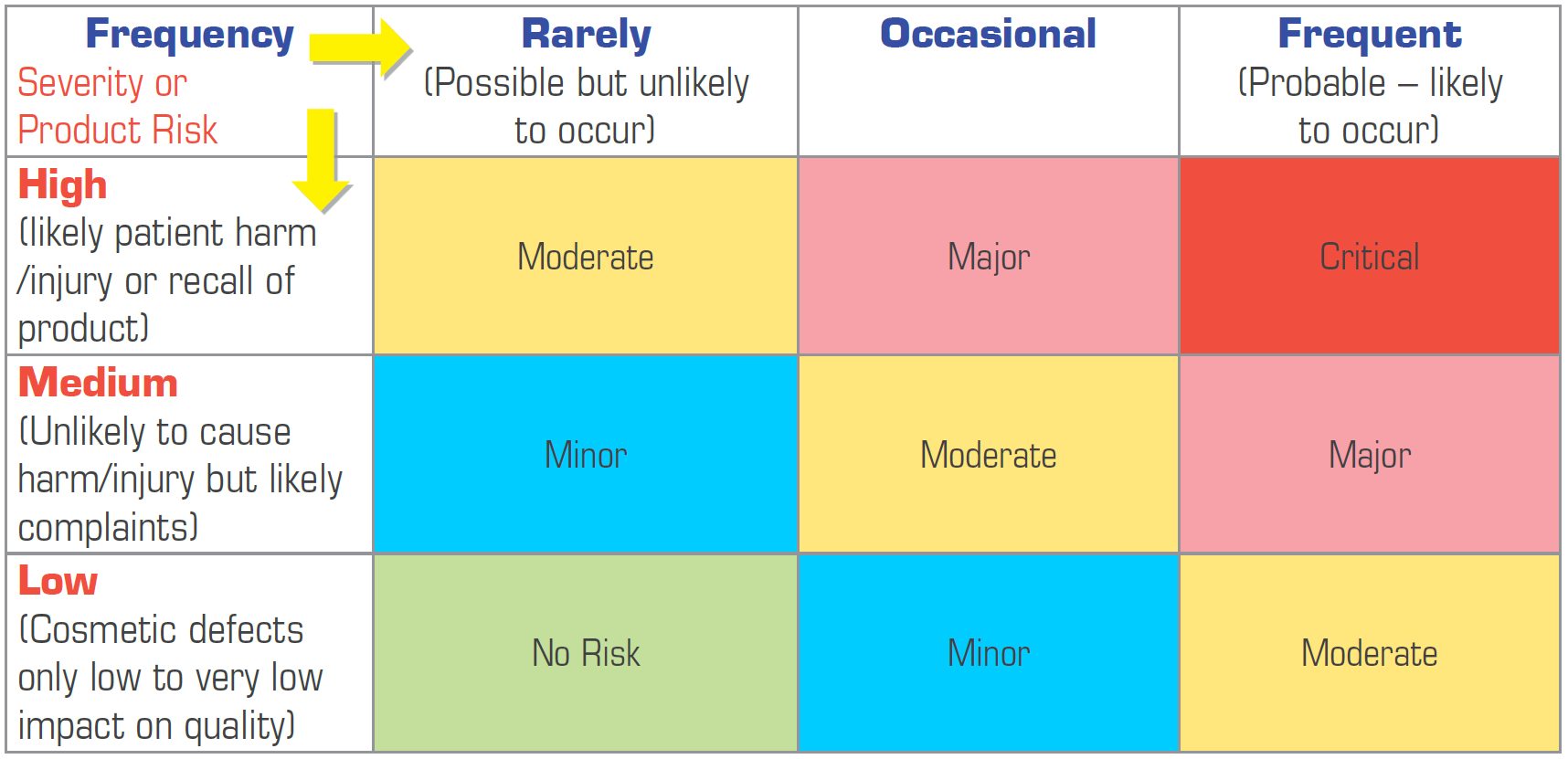
The stages required for applying ISO 14971 principles to risk management for medical devices can be typically broken into 6 steps:
GMP Coaching Program Assists Biopharmaceutical Manufacturer
November
11,
2016
A biopharmaceutical manufacturer engaged SeerPharma to provide on-going coaching to their Quality personnel and advise on improvements to help prepare for a FDA inspection.