With the ever-evolving advancements of computerised systems and their involvements in the pharmaceutical, medical device, biotechnology and clinical trial industries, it is more critical than ever to ensure that these systems are appropriately assessed, validated and controlled. This is essential to safeguard the ensuing data integrity, product...
Webinar | AI in Life Sciences: Promise and Risk
Artificial Intelligence (AI) is reshaping industries worldwide, and life sciences is no exception. From drug discovery to clinical decision support, AI has the potential to transform the development, testing, and delivery of therapies. Yet, while the promise is immense, adoption remains cautious. The road ahead requires striking a balance between...
As a life sciences manufacturer, there’s mounting pressure to integrate artificial intelligence (AI) across the product lifecycle and capitalize on the opportunities this technology presents to the industry. Organizations face the complex challenge of balancing innovation with regulatory compliance.
Safe and effective adoption of AI can require...
Computer Software Assurance - Configuration, Design & Closing the Loop
In the previous article, we looked at including Test Cases and the Functional Risk Assessment into the Functional Requirements Specification. This provided multiple benefits over maintaining these as separate documents:
- Forces requirements to be testable (described in the test cases)
- Forces thinking using ‘what if’ scenarios on requirements which...
Computer Software Assurance (CSA) - Use of Critical Thinking
In previous articles, we looked at reasons to rethink and simplify the approach to Computer System Validation (CSV). This highlighted a system level ‘macro risk’ assessment using several key factors (including GAMP category and direct/indirect product impact) to determine an overall Risk Profile Score; and then a GAMP functional risk assessment...
Computer Software Assurance (CSA) – Risk and Reward
In a previous article, we looked at the reasoning behind why the FDA thought there was a need for a change of approach for many companies with their Computer System Validation (CSV). In this article we will look at how a more structured risk assessment approach will lead to greater computerised system rewards – and at a lower cost.
Application of ISO 14971 Risk Management to New Medical Devices
The stages required for applying ISO 14971 principles to risk management for medical devices can be typically broken into 6 steps:
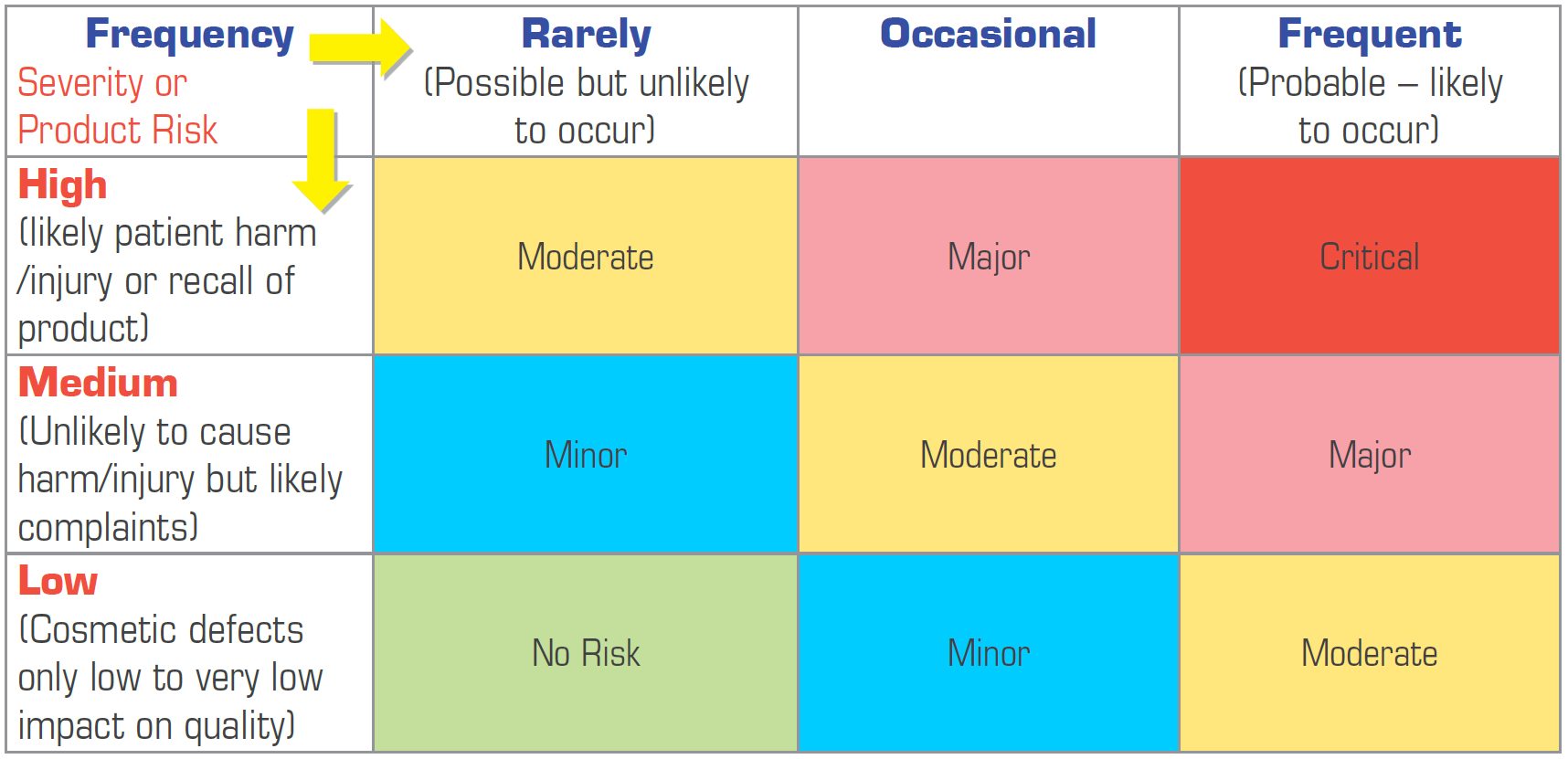
Pharmaceutical Quality Risk Management in GMP Compliance
Quality Risk Management (QRM) is a GMP compliance requirement for Pharmaceutical organisations. The ICH Q9 guideline on Quality Risk Management (Annex 20 of the PIC/S GMP Guide) is expected to be adopted by manufacturers to ensure compliance with clauses 1.12 and 1.13 of Part I of the PIC/S GMP Guide:
SeerPharma Sponsors BioMelbourne “Corporate Culture and Risk” Session
As part of our commitment to the local biotech sector, SeerPharma was proud to sponsor a BioMelbourne Network session on Corporate Culture and Risk earlier this year.
GMP Coaching Program Assists Biopharmaceutical Manufacturer
A biopharmaceutical manufacturer engaged SeerPharma to provide on-going coaching to their Quality personnel and advise on improvements to help prepare for a FDA inspection.