The latest revision of ICH E6 (R3), finalised in January 2025 and enforced in Europe since 23 July 2025, marks a significant shift in clinical trial governance, moving beyond incremental updates to reflect modern trial practices and technologies. The guideline emphasises quality by design, risk-based proportionality, and clearer delineation of...
Recurring deviations—especially those involving the same or similar issues—are often red flags for regulatory inspectors. In many cases, significant inspection deficiencies related to inadequate corrective actions could have been prevented if the organisation had recognised the patterns earlier and taken proactive steps.
In industries where compliance is critical such as pharma, biotech, and medical devices, the way we train our workforce has real-world consequences. It affects not just audit outcomes, but also operational safety, product integrity, and ultimately, patient wellbeing.
Data Integrity failures remain one of the most common reasons for regulatory action in the pharmaceutical industry and continue to be among the most frequently cited findings in inspections globally. Notably, approximately 60% of these failures could be avoided with the implementation of robust data governance frameworks.
In this actionable webinar for quality leaders, we’ll reveal essential automation strategies and how to configure CAPA processes to match your unique business processes—without custom coding or IT dependencies. Learn how forward-thinking companies are leveraging configurable quality event management systems to dramatically reduce CAPA resolution...
For more than 15 years, SeerPharma has proudly collaborated with the Malaysian Organisation of Pharmaceutical Industries (MOPI) to design and deliver regular Good Manufacturing Practice (GMP) training programs for pharmaceutical and medical device manufacturers across Malaysia. These training sessions are endorsed by, and frequently attended by,...
Explore the critical role of digital transformation in asset management—an often overlooked but vital function for life sciences companies producing regulated products.
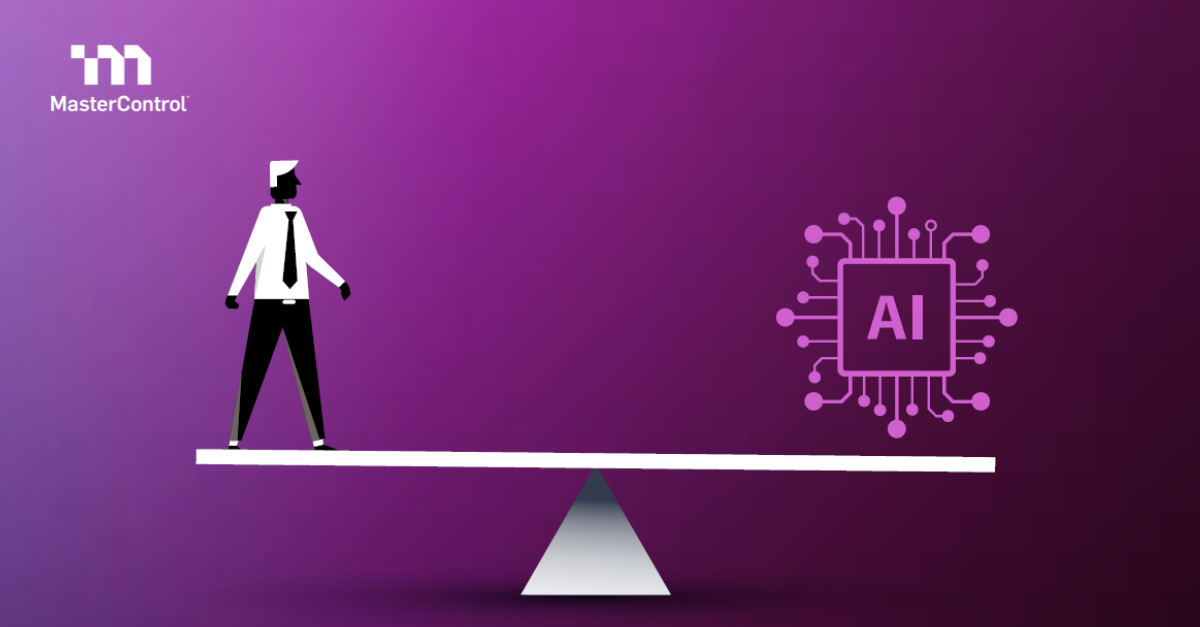
As a life sciences manufacturer, there’s mounting pressure to integrate artificial intelligence (AI) across the product lifecycle and capitalize on the opportunities this technology presents to the industry. Organizations face the complex challenge of balancing innovation with regulatory compliance.
Safe and effective adoption of AI can require...
The Parenteral Drug Association (PDA) is a leading global provider of science, technology and regulatory information and education for the pharmaceutical and biopharmaceutical community.
SeerPharma Senior GMP Consultant Michelle Peake has been invited to speak at PDA Annex 1 Implementation: Inspection Ready Workshop 2025, 17 - 18 June 2025 in ...

_Webinar_Sep2025_Promo.png)