INFOGRAPHIC: A snapshot of how SeerPharma supported its clients in 2019 on matters of Quality Assurance and GMP compliance.
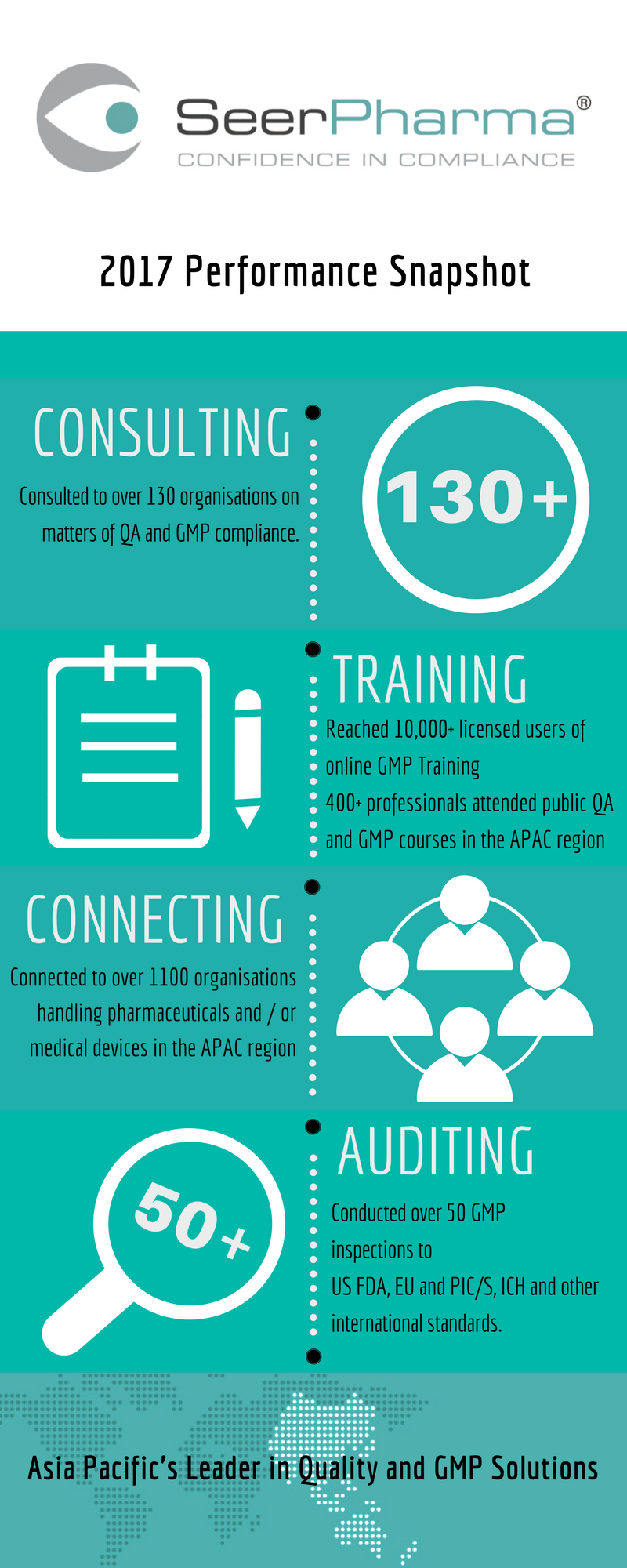
Infographic: QA & GMP Compliance Performance Snapshot for 2017
INFOGRAPHIC: A snapshot of how SeerPharma has supported its clients in 2017 on matters of QA and GMP compliance.
INFOGRAPHIC: A snapshot of how SeerPharma has supported its clients in 2016 on matters of QA and GMP compliance.
CSV Gap Assessment for Pharmaceutical Distributor
A major pharmaceutical distribution organisation engaged the services of SeerPharma (Singapore) Pte Ltd to provide a review on the compliance of its core SAP-Centric solution to the GxP Computer Systems Validation (CSV) requirements of both FDA 21 CFR Part 11 and PIC/S Guide to GMP Annex 11. There were 2 phases to this project.
Pharmaceutical Manufacturers Association Recommends G(QC)LP Services
One of our senior consultants in Singapore, received the following glowing recommendation from the Sri Lanka Pharmaceutical Manufacturers' Association (SLPMA) which is a testament to the quality of our team in Singapore who continually strive to deliver exceptional service to customers in the region.

.png)