INFOGRAPHIC: A snapshot of how SeerPharma supported its clients in 2019 on matters of Quality Assurance and GMP compliance.
Kalbe Farma is a leading healthcare provider in Indonesia, with extensive operations throughout the South East Asian region. Its’ subsidiary PT Kalbio Global Medika (KGM) has recently opened a new 11,000 square meter factory in Indonesia, which received a Honourable Mention for the 2017 Facility of the Year Awards from ISPE.
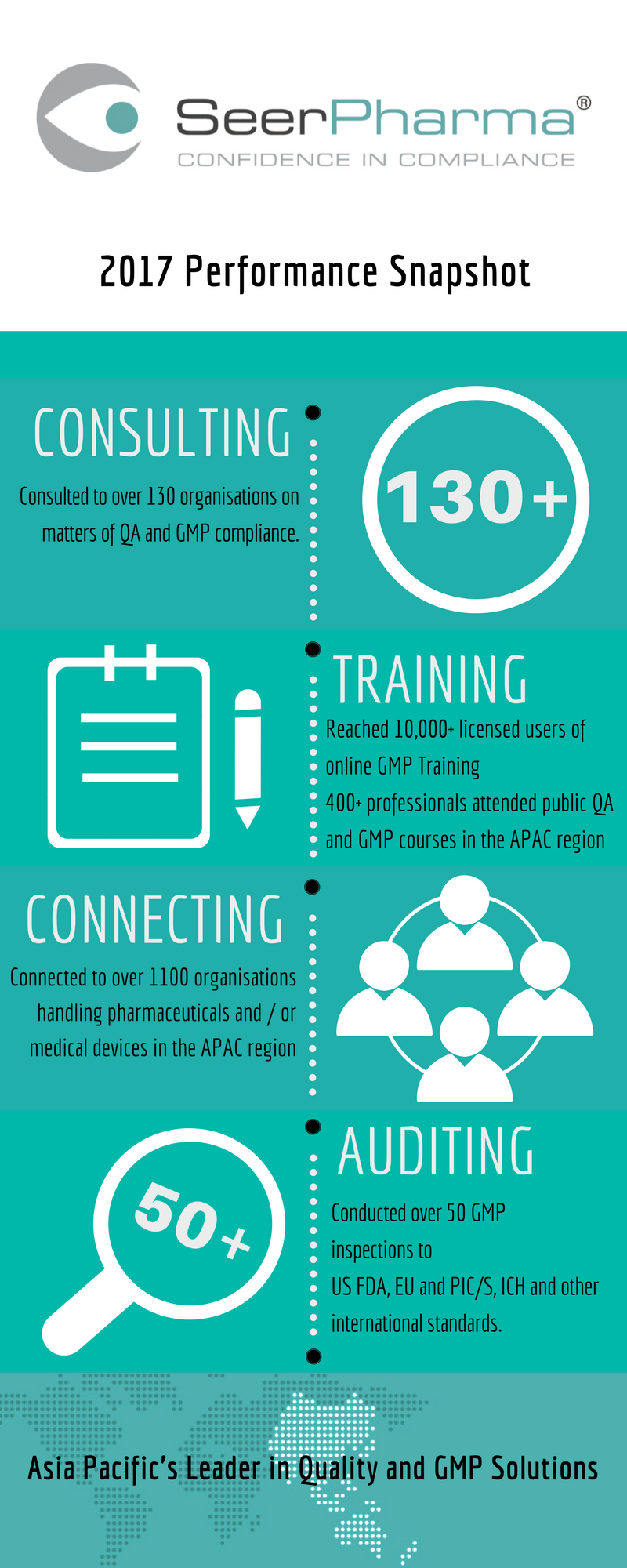
Infographic: QA & GMP Compliance Performance Snapshot for 2017
INFOGRAPHIC: A snapshot of how SeerPharma has supported its clients in 2017 on matters of QA and GMP compliance.
INFOGRAPHIC: A snapshot of how SeerPharma has supported its clients in 2016 on matters of QA and GMP compliance.
Cell Therapies Training with CRC for Cell Therapy Manufacturing
Cell therapies are an emerging form of healthcare that have started to produce transformative outcomes for patients with previously refractory diseases, but they are substantially more complex than small molecule or biologic drugs and present significant challenges for researchers and companies in cell therapy translation.
GMP Coaching Program Assists Biopharmaceutical Manufacturer
A biopharmaceutical manufacturer engaged SeerPharma to provide on-going coaching to their Quality personnel and advise on improvements to help prepare for a FDA inspection.
GMP Data Integrity Masterclass
GMP Data Integrity is an issue that a number of our clients have approached us about recently. The concept of Data Integrity has been around for quite some time, with the TGA introducing the concept of electronic records in to the previous Australian code of GMP back in 1990, while the FDA made 21 CFR Part 11 Electronic records and signatures...
Pharmaceutical Manufacturers Association Recommends G(QC)LP Services
One of our senior consultants in Singapore, received the following glowing recommendation from the Sri Lanka Pharmaceutical Manufacturers' Association (SLPMA) which is a testament to the quality of our team in Singapore who continually strive to deliver exceptional service to customers in the region.
Good Distribution Practice (GDP) Training in Asia
Zuellig Pharma is Asia’s leading healthcare service provider offering a broad range of innovative services including distribution of pharmaceuticals, medical devices, special solutions and other healthcare related consumer products.
Effective CSV in the Pharmaceutical / Medical Device Industry
You’re a Quality, IT, or Operational manager working for a Pharmaceutical / Medical Device company, responsible for the computer systems used in-house. Working in the Pharmaceutical / Medical Device industry, means that regulators such as the FDA and TGA will scrutinise the use and validation of these business critical systems.

.png)