SeerPharma is thrilled to welcome the 2021 Spring cohort of students studying Good Manufacturing Practice (GMP) at the University of Technology Sydney. This cohort is a global blend of graduates and industry professionals. Our investments with migrating this program online over several years has placed us in a strong position through the COVID-19...
Research and operations of Medical Technology, Biotechnology and Pharmaceutical (MTP) companies aligned with and in some cases certified to Quality Management System (QMS) Standards will ensure Australia is at the forefront of medical innovation.
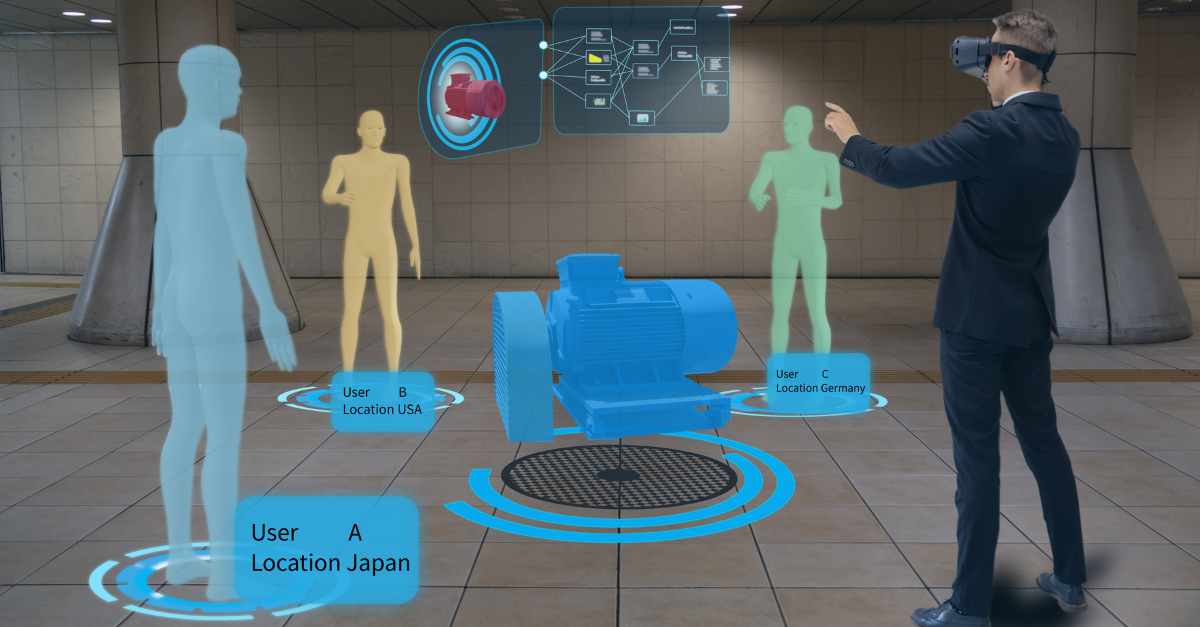
Validation 4.0 – A Near Future State
ISPE recently hosted a webinar on Validation 4.0 and where it sat within their ISPE Pharma 4.0TM Operation Model. It was with no surprise that an audience survey rated ‘documentation’ as the biggest hurdle / constraint to validation.
Chemika is an independently owned chemical analysis laboratory located in Girraween, Sydney, and is licensed with the Therapeutic Goods Administration (TGA) and the Australian Pesticides and Veterinary Medicines Association (APVMA). Chemika provide chemical testing services to manufacturers of human therapeutics and veterinary medicine. Services...
Meet Mario Cano Gorra - Master of GMP Student at UTS
I am a Mexican-born citizen. Since elementary school, I’ve displayed a natural knack for biology, physics, and mathematics. Naturally, I studied a Bachelor of Science in Pharmaceutical Engineering in one of the largest and most prestigious universities in Mexico, the National Polytechnic Institute. Whilst studying I met my wife, and we have been...
Let’s just say what we’re all thinking: 2020 was rough. It threw pretty much every element of our lives into disarray, and we suddenly had to become experts at navigating a new way of working, schooling and socialising. It was disruptive, and it was challenging, but it was also transformative. While the past 12 months might have felt like 12...
In the previous 2 articles (linked below) we have looked at what the Quality Management System (QMS) of the near future (Quality 4.0) may look like under MTP 4.0 (Industry 4.0 for the Medical Technology, Biotechnology and Pharmaceutical sector).
Recall that, MTP 4.0 is a holistic operating model that incorporates digital technologies, data...
In a previous article we looked at the emergence of MTP 4.0 (Industry 4.0 for the Medical Technology, Biotechnology and Pharmaceutical sector) as a holistic operating model to facilitate an agile development and manufacturing environment for the near future.
Being holistic, the operating model covers all aspects of the business, using a digital...
SeerPharma is excited to announce Harsha Gupta as the inaugural recipient of the SeerPharma Scholarship. Harsha is currently enrolled in the C04301 Master of Good Manufacturing Practice (GMP) at UTS and works as a Senior Manager, Manufacturing and Process Development for a company that manufactures allogenic Mesenchymal Stem Cells (MSCs). Prior to...