SeerPharma's induction and refresher Good Manufacturing Practice (GMP) training solution started as 9 modules on a CD. With the dot com boom, the product was redeveloped from scratch and launched from an online Learning Management System (LMS) to adopt more modern and functional technology. It grew from there, with 40 modules now available across...
SeerPharma was invited by the Stevanato Group and Lonza to present on Pharmaceutical Quality Management System (QMS) Certification at The Innovative Solutions for Pharmaceutical Packaging Roadshow in Singapore.
Kalbe Farma is a leading healthcare provider in Indonesia, with extensive operations throughout the South East Asian region. Its’ subsidiary PT Kalbio Global Medika (KGM) has recently opened a new 11,000 square meter factory in Indonesia, which received a Honourable Mention for the 2017 Facility of the Year Awards from ISPE.
Following a successful pilot program conducted in 2015-16, the Therapeutic Goods Administration's (TGA's) Pharmacovigilance Inspection Program (PVIP) has been implemented as an initiative to help sponsors of medicines to meet their pharmacovigilance obligations.
SeerPharma is helping sponsors prepare for the inspections.
SeerPharma to Present on Quality Management System Certification
SeerPharma have been invited by the Stevanato Group and Lonza to present on Pharmaceutical Quality Management System Certification at The Innovative Solutions for Pharmaceutical Packaging Roadshow in Singapore.
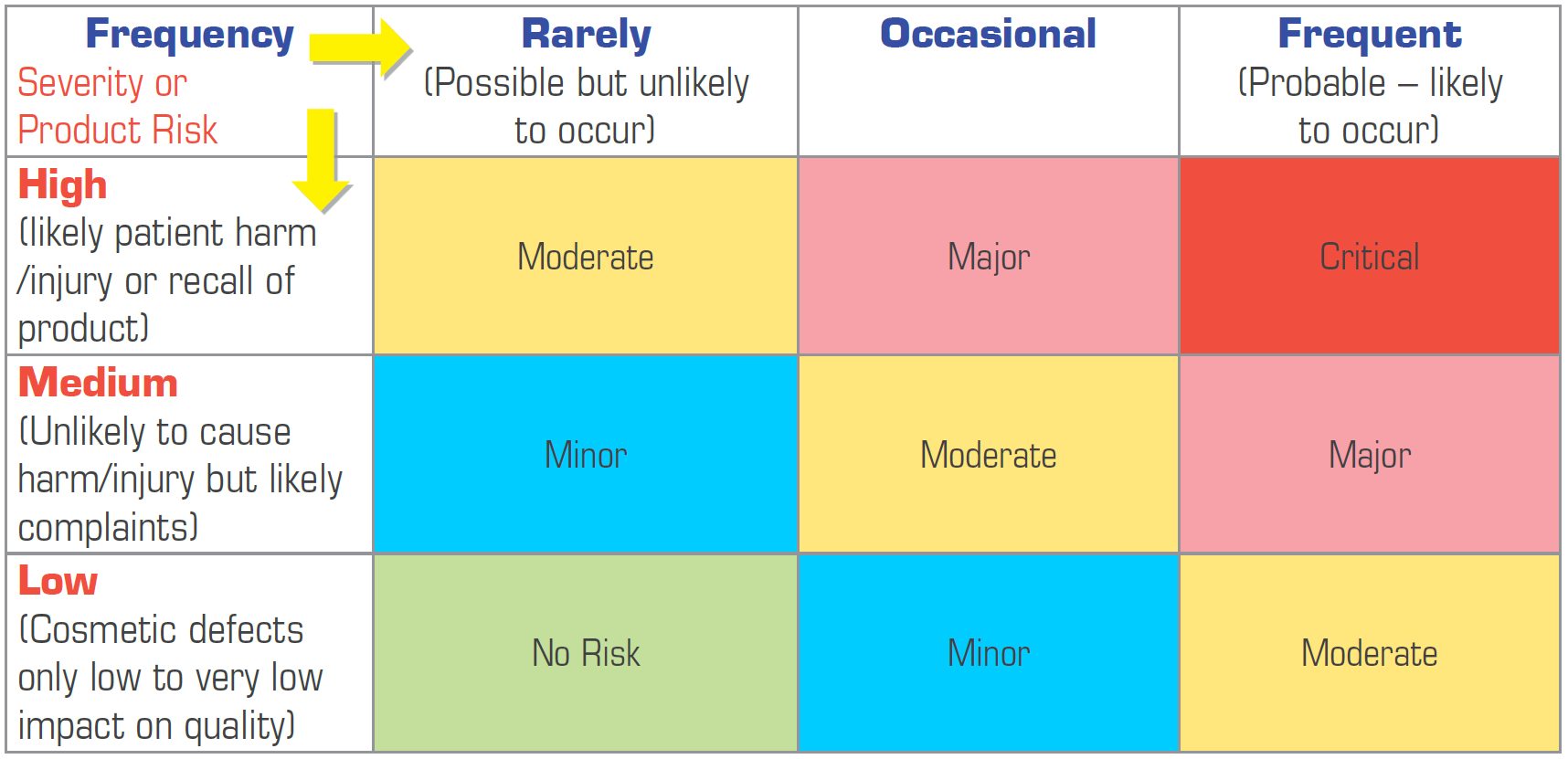
Application of ISO 14971 Risk Management to New Medical Devices
The stages required for applying ISO 14971 principles to risk management for medical devices can be typically broken into 6 steps:
Experience from Attending Master of GMP Course at UTS
UTS International student Tong Zhao is a Pharmacy graduate from Liaoning Province, China, currently undertaking the Master of Good Manufacturing Practice here in the UTS Graduate School of Health. We sat down with Tong to hear her story.
Computer System Validation: Starting on the Right Track
Most of us follow a professional sporting team. Each year we start the season with hope and enthusiasm that ‘this year’ will be ‘our year’. Have our recruiters and management assembled the right mix of youth and experience and will they be coached to their strengths? Is the framework in place to raise our odds of success ?
Developing an Advanced Manufacturing Process
Australian biotech company VivaZome Therapeutics has announced that it has been awarded an Australian Government CRC-P grant of $2.18m in support of the project “Enabling Exosome Therapy: Developing an Advanced Manufacturing Process”, with the funds provided under the Advanced Manufacturing Scheme.
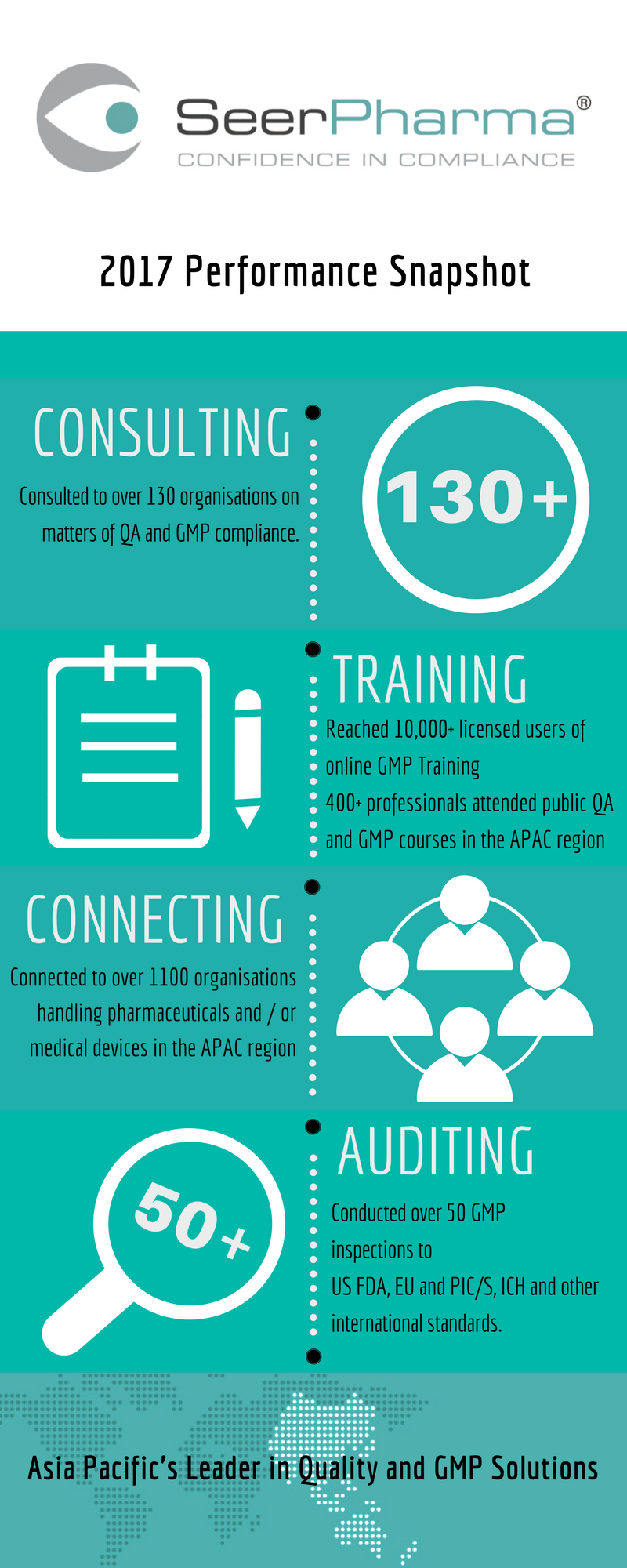
Infographic: QA & GMP Compliance Performance Snapshot for 2017
INFOGRAPHIC: A snapshot of how SeerPharma has supported its clients in 2017 on matters of QA and GMP compliance.