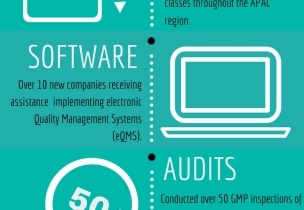
INFOGRAPHIC: A snapshot of how SeerPharma has supported its clients in 2016 on matters of QA and GMP compliance.
Pharmaceutical Quality Risk Management in GMP Compliance
Quality Risk Management (QRM) is a GMP compliance requirement for Pharmaceutical organisations. The ICH Q9 guideline on Quality Risk Management (Annex 20 of the PIC/S GMP Guide) is expected to be adopted by manufacturers to ensure compliance with clauses 1.12 and 1.13 of Part I of the PIC/S GMP Guide:
Effective Management of Risk-based GMP for Health Products
We're excited to announce the first list of confirmed speakers for our Symposium to be held in Singapore on 16 - 17 March 2017: A Global Perspective on Effective Management of Risk-based GMP for Health Products.
SeerPharma Exhibiting at BioPharma Asia 2017
SeerPharma is excited to be sponsoring and exhibiting at BioPharma Asia from the 21st – 23rd March at the Suntec Convention Centre in Singapore.
Manufacturer Turns to SeerPharma for Pharmaceutical QA Manager
A multinational pharmaceutical contract manufacturer recently required the assistance of a senior pharmaceutical QA resource to assist with managing Quality and GMP issues at their manufacturing site.
Cell Therapies Training with CRC for Cell Therapy Manufacturing
Cell therapies are an emerging form of healthcare that have started to produce transformative outcomes for patients with previously refractory diseases, but they are substantially more complex than small molecule or biologic drugs and present significant challenges for researchers and companies in cell therapy translation.
Electronic Quality Management System (eQMS) for Pharmaceutical Company
SeerPharma’s Software Solutions team has been engaged to implement an electronic Quality Management System (eQMS) for a major multinational pharmaceutical company.
SeerPharma Sponsors BioMelbourne “Corporate Culture and Risk” Session
As part of our commitment to the local biotech sector, SeerPharma was proud to sponsor a BioMelbourne Network session on Corporate Culture and Risk earlier this year.
EU GMP and FDA GMP Audit
SeerPharma was asked to conduct a mock GMP audit of a Korean Pharmaceutical company that produces non-sterile solid dosage forms and finished products.
GMP Supplier Audits in Europe and Asia
A number of firms have requested our expertise to provide technical and compliance audits of contract manufacturers that they currently use in Europe and Asia.