In a previous article we looked at the emergence of MTP 4.0 (Industry 4.0 for the Medical Technology, Biotechnology and Pharmaceutical sector) as a holistic operating model to facilitate an agile development and manufacturing environment for the near future.
Being holistic, the operating model covers all aspects of the business, using a digital transformation as a driver for change.
Quality Management has often been seen as a bottleneck for change, so implementing a more streamlined quality approach, whilst still meeting regulatory compliance, will be the challenge to overcome.
This article looks at the Customer Complaints, CAPA, Deviations, Document Control and Training subsystems with a QMS and explores what these may look like under Quality 4.0.
Customer Complaints
Customer complaints and feedback can be hampered by the lack of initial information provided about the issue and the turnaround time taken to resolve the issue.
As each saleable item becomes serialized (tagged and tracked from manufacture to end customer), and by creating a simple ‘Complaint Portal’ where entering the item tag ID will automatically provide the company with a full trail of manufacturing, distribution and related item data, system analytics can be automatically performed based on the other data gathered by the complainant.
By simplifying the initial data gathering process (perhaps with Artificial Intelligence and Natural Language Processing based off a history of data needed for each type of complaint and in the ability to auto categorise a complaint), automatic feedback can be provided and the complaint either automatically closed or instantly escalated to a relevant company representative.
Constant analysis over all complaints can be used to auto-adjust the response approach and automatically trigger other changes or corrective actions over affected areas of the process or product CQAs and CPPs.
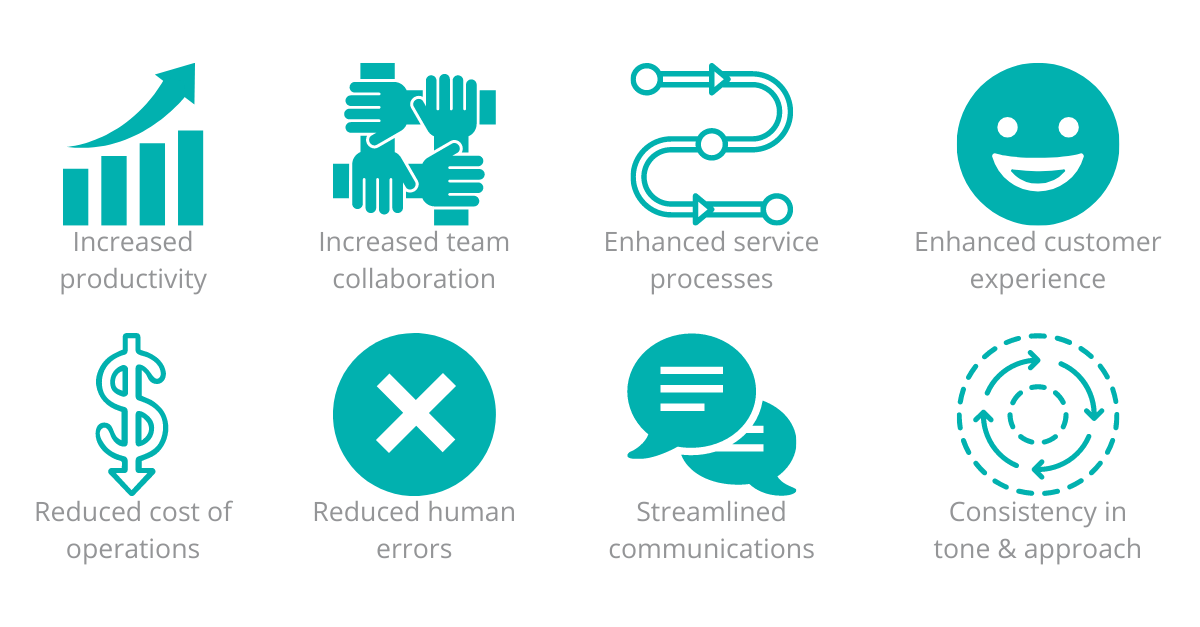
The benefits of automating the customer complaints process will be seen by both the customer and the respondent company.
Figure 1 – Benefits of an automated customer complaints process
CAPA (Corrective and Preventive Action)
By definition, CAPAs are both reactive (corrective) and proactive (preventive). In the maintenance world, an effective program has the ratio of preventive to corrective actions at about 6:1.
Preventive is certainly better than corrective action, but there is still no guarantee that you may be performing unnecessary operations, or operations far earlier than they are really needed.
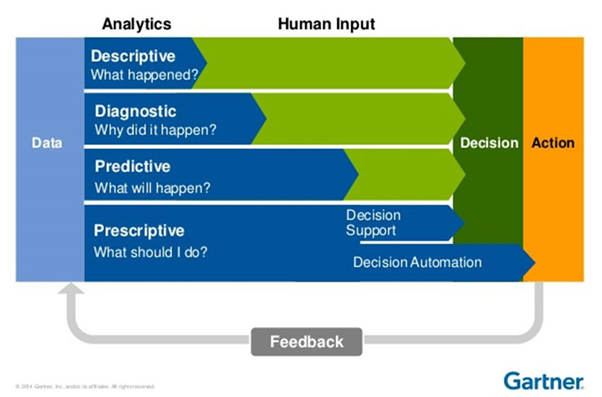
Under Quality 4.0, the aim is to replace both corrective and preventive actions with predictive and prescriptive actions as much as possible. As more and more historical data is gathered about equipment, processes, people and materials, this can be used to predict future and ‘near future’ events and either inform on potential situations or automatically take action in real time.
Even with the best of environments, emergency work will always be necessary. With integrated processes and systems, downtime can be minimised and process line reconfiguration automated.
Much of the CAPA reporting can be automated, with eQMS CAPA actions receiving data directly from the process itself (equipment or controlling system) with human confirmations.
Longer term CAPA verification confirmations can also be automatically sent from the process itself to either close of readdress the CAPA.
Figure 2 – With detailed analytics, CAPA human input will be minimised
Deviations
OAs more and more process actions become predictive and prescriptive, there should be fewer deviations that require manual interaction.
In saying that, equipment breakdowns, human error, raw material failure, process interruptions, etc will never be able to be totally eliminated.
The MTP 4.0 push will be to ensure that any downtime is minimised by coordinating upstream and downstream actions to work around the deviation as much as possible.
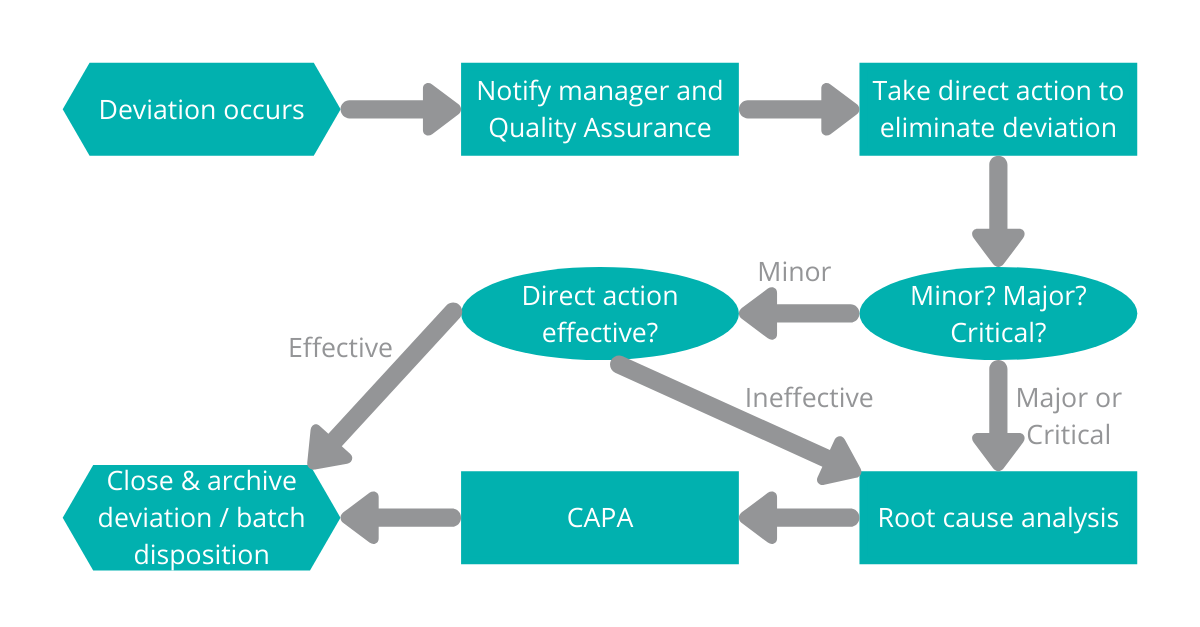
The Quality 4.0 push will be to automatically capture as much relevant deviation data as possible, analyse the deviation against the data / previous history and ensure that the process is functional in the shortest timeframe. Deviation cause, effect and rectification needs to be communicated back into the data pool and to any relevant stakeholders.
Event escalation triggers will automatically determine whether any additional root cause analysis and CAPA/predictive actions are required.
Figure 3 – The time between the deviation occurring and closing can be minimised
Document Control and Training
Although Document Control and Training are separate systems, when it comes to the QMS and a Quality 4.0 framework, these may look quite different than they currently do in many companies.
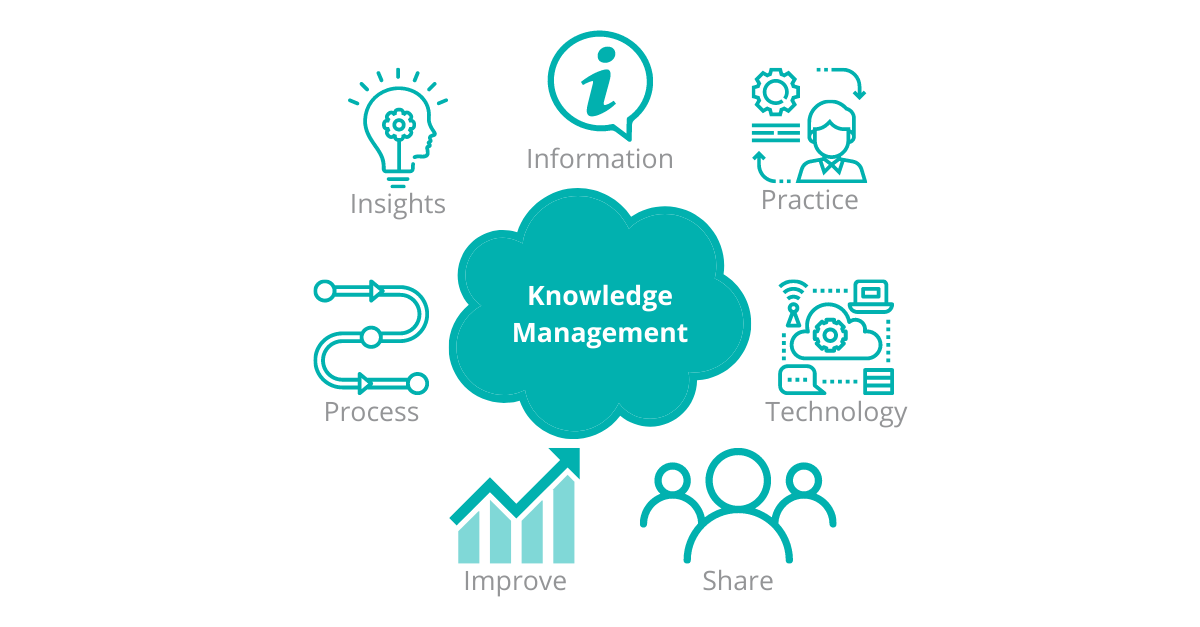
Rather than a ‘push, read and acknowledge’ loop, companies will use a true ‘Knowledge Management’ approach; allowing knowledge ‘users’ to both be ‘pushed’ relevant content and ‘enrol in and pull’ requested and relevant content.
With detailed process maps, users can be ‘tagged’ against processes and parts of processes they need to be anything from ‘expert at’, to need a level of ‘fundamental awareness’. This can either be an explicit association or implicitly by job role or function.
Mandatory content can be pushed, and actual operation of the process can be prevented or restricted until competency is demonstrated based on a direct interface between the process systems and the training database.
By allowing users to ‘rate and assess’ the content they receive as part of a feedback loop, the content itself can be reviewed, updated and enhanced to make it more appropriate for the end users.
As additional knowledge content is loaded, it can be tagged against relevant processes, so that over time, and using Natural Language Processing (NLP) and Machine Learning (ML), the system learns about the content and is able to auto tag it.
To enable this to happen, as a first step, knowledge content will need to be broken down into smaller, task specific items that can be either requested on demand or pushed to individuals based on the up-to-date training matrix known by the system and compared to the up-to-date process maps and related content.
In this way, training can be automatically tailored to the individual. Knowledge content changes, depending upon need and criticality, will be able to be conveyed via a communication app based on each individual’s availability and circumstance.
As each individual will be assigned more relevant content (and removed or become an optional attendee of less relevant content), training key performance indicators (KPIs) will be far more accurate and meaningful than they currently are.
Figure 4 – Knowledge management provides an on-demand and as-needed repository of content
Summary
With better knowledge gained from vast amounts of data gathered during the development, manufacture and actual use of products, and the subsequent analysis available on this, there should be fewer deviations and customer complaints. The amount fewer is still debatable.
Whatever the number, better knowledge will lead to faster response times, more knowledgeable users and a more agile QMS to support a more agile operating environment.
Figure 5 – Overview of possible changes under Quality 4.0 for specified subsystems
| System | Current | Quality 4.0 |
| Customer Complaints | • Sub optimal communication / feedback loop times / customer service • Lack of visibility between complaints • Reactive KPIs / reports |
• Each saleable item is serialised for tracking • Real time entry and analysis of complaints • Automatic feedback /escalation to appropriate resources • Real time reports / change /CAPA triggers • Better customer service |
| CAPA | • Reactive for correction actions • Preventive actions occur whether needed or not • Downtime affects production • Lack of visibility between actions, dependencies and timeframes |
• Fewer corrective actions and more predictive actions • Real time feedback loops for auto corrections • Integrated with other quality and manufacturing processes • Auto line reconfiguration to minimise downtime • Auto escalations to enforce action timings and dependencies |
| Deviations | • Reactive based on occurrence • Affects upstream and downstream processes • Manual notification, investigation and delay on remediation |
• Less deviations as systems monitor conditions and warn / auto adjust • Minimises effects on upstream / downstream processes • Auto notification with streamlined remediation processes |
| Document Control / Training | • Documents pushed to large groups in a batch operation • Not all documents are 100% relevant to all who are required to read • Lack of feedback loop for those asked to read • KPIs report a ‘point in time’ snapshot of documents read • Data fed to users not necessarily at the time they need it • No integration between competency and operation at the time of operation |
• Knowledge content will be more tailored per process • Knowledge relevance will be tailored per individual • Push and pull content available • Individuals will be able to rate content as part of an improvement loop • KPIs will better reflect the situation • The system will auto adjust using machine learning per individual • Systems will auto advise individuals on specific operation at the time of the operation based on competency |
Related Quality Management under MTP 4.0 articles:
ISPE Pharma 4.0TM – is a trademark registered with the European Union Intellectual Property Office and owned by the International Society for Pharmaceutical Engineering, Inc.
