Industry 4.0, or the Fourth Industrial Revolution is a term used to describe the use of smart technology in manufacturing and industrial processes.
ISPE (the International Society for Pharmaceutical Engineering) has trademarked the term ‘ISPE Pharma 4.0’ to cover Industry 4.0 in the life sciences sector.
As the Industry 4.0 concept is equally applicable to all the life sciences sector, we are using the term ‘MTP 4.0’ (Medical Technology, Biotechnology and Pharmaceuticals) to cover this.
MTP 4.0 is a holistic operating model for factories and supply chains of the future based on Industry 4.0 capabilities, digital maturity, data integrity and restructured organisational process control.
In simple terms, it aims to prepare ‘smart operators for smart factories’.
As seen in Figure 1, MTP 4.0 spans almost every area of the organisation, and this series of 3 papers discusses the Quality 4.0 systems covered under the Holistic Control Strategy.
This article discusses an introduction to MTP 4.0 and looks at Audits and Change Control.
Another article discusses Safety Incidents, Suppliers, Materials and Risk Management.
Figure 1 – ISPE Pharma 4.0TM Operating Model
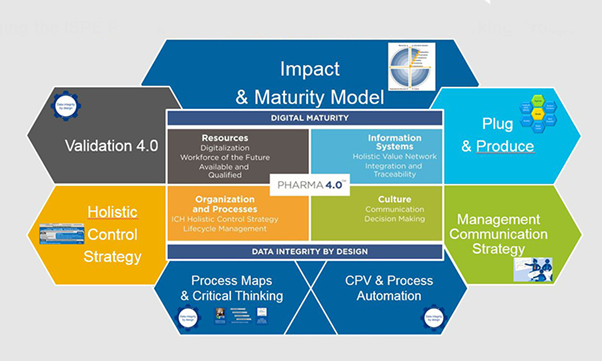
Source: https://ispe.org/initiatives/pharma-4.0
There are several Maturity Models that rank individual companies from simple computerisation (i.e. simplifying repetitive manual tasks) all the way to full adaptability (i.e. where the systems themselves control the process).
Depending upon each company’s current level of maturity in each facet of the transformation, and its willingness to change, the journey to reach both predictive (i.e. able to notify before an event occurs), and adaptive (i.e. able to automatically take action and avoid events and reconfigure the surrounding processes to continue) processes may be long and arduous.
Having a defined MTP 4.0 strategy is a not a mandated position, but as new digital technology emerges and develops, companies that have not set the framework for easy adoption, will be at a distinct disadvantage to those that have.
As our current reactive processes become more predictive and then adaptive, our quality management systems will also adapt.
In the future there will be far fewer manually created documents as the role of quality moves from actions that may slow down or even stop continuous process manufacturing to a more inline continuous quality assurance.
In line with that, rather than being a siloed department, QA professionals will be part of multi-disciplined teams with a common goal of consistently producing safe and effective products.
As the ICH Q10 Guideline (Pharmaceutical Quality System) sets a framework for quality risk management (ICH Q9 – Quality Risk Management), both in drug development (ICH Q8 – Pharmaceutical Development and ICH Q11 – Development and Manufacture of Drug Substances (chemical entities and biotechnological/biological entities)) and in the commercial phase of the product lifecycle (ICH Q12 – Product Lifecycle Management), Pharma 4.0 enhances these guidelines with a Holistic Control Strategy.
Figure 2 – ISPE Pharma 4.0 - enabled and ICH-enabled holistic control strategy
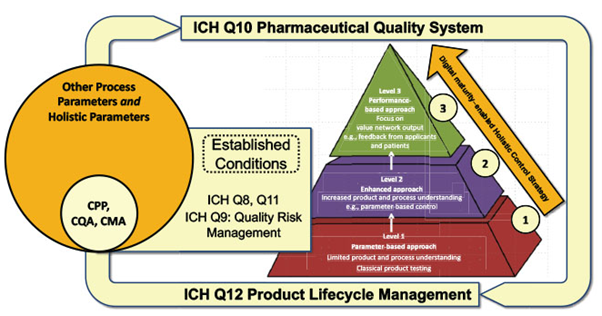
CPP – Critical process parameters, CQA – Critical quality attributes, CMA – Critical material attributes
Source: ISPE Engineering / January/February 2020 / Applying Holistic Control Strategy in Pharma 4.0™
This series of papers looks at some of the traditional quality subsystems (covered in ICH Q10) and what they may look like under Quality 4.0.
Audits
Auditing has typically been a reactive process to verify (through inspection or examination) that defined processes are being followed.
It checks that the controls are in place to ensure that processes remain in compliance with standards and that these controls are being followed and that they are effective.
Under Quality 4.0, manual auditing will be a simpler review of individual system / process / product auto generated reports. Systems themselves will be able to provide control reports that show that system is functioning as expected.
If all processes and their Critical Control Attributes (CQA) are well defined, and Critical Process Parameters (CPP) are being measured, controlled and integrated with upstream and downstream related processes, the systems themselves will be able to provide the Continued Process Verification (CPV) needed to ensure that products are manufactured under controlled conditions.
With Vision Technology able to monitor, detect and record unexpected events, these can automatically notify or initiate deviations.
To ensure data integrity, key recorded results can be tracked using a blockchain ledger.
Audits on the quality management system itself will consist of a review of the controls in place as described in each subsystem below and will be able to be cross referenced from within an electronic Quality Management System (eQMS).
Figure 3 – Computer Aided Audit Techniques
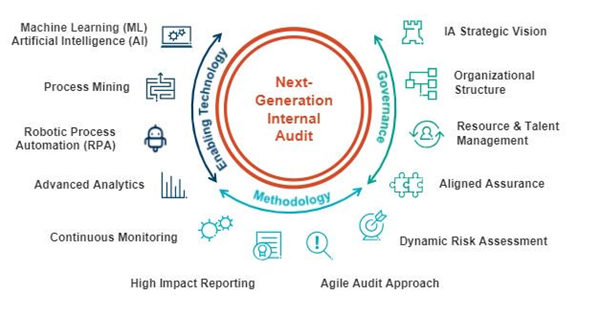
Source: https://blog.protiviti.com/2019/03/13/youve-heard-of-next-gen-internal-auditing-heres-a-look-inside/
Change Management
Once all the company’s processes, products and data maps are defined, all changes will be reflected in a change to one or more of these.
As the overall management structure will need to be less of a formal hierarchical and siloed structure (as many companies currently are) and more of an agile ‘banding and disbanding’ of defined teams (still with individuals specifically skilled and experienced in each facet of the business), this responsibility matrix will need to be maintained over the control and responsibility of each area of the maps.
Maintaining an immutable audit trail over these process and data maps, and the current and historical control structure, with references back to the reason for change, will provide a well-managed change management process.
As each change itself consists of both a management control process (planning, defining actions and verifying the outcome) and individual actions, using an eQMS to track and escalate activity timeframes will provide control over the human actions.
Organisations in the final phase of MTP 4.0 maturity (adaptability), will have systems that can automatically change based on Machine Learning algorithms analysing more extensive data sets. These changes will still need to be formally recorded, reviewed and accepted.
Figure 4 – With ‘adaptability’, systems will be able to self-optimise
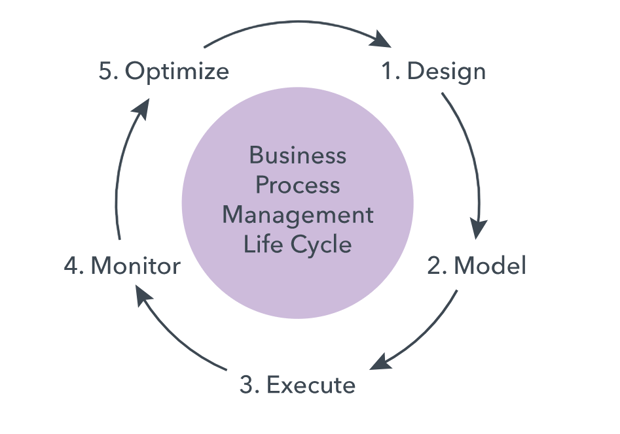
Source: https://www.lucidchart.com/pages/business-process-mapping
Summary
In future articles we will explore other quality management subsystems, but what is clear, as companies better understand and embrace the ‘holistic’ changes to all areas of the business, Quality 4.0 approaches will need to be integrated with these changes.
Figure 5 – Overview of possible changes under Quality 4.0 for Audits and Change Management
| System | Current | Quality 4.0 |
| Audits | • Manual review • Reactive process to verify controls • Manual or semi-automated QMS |
• Automatic / semi-automatic review of control • Inline controls designed into each process • Review process and exception reports • Integrated eQMS |
| Change Management | • Several different processes per business area • Lack of integration across the organisation • Vested interests across siloed business areas • Lack of visibility across all changes |
• References centralised process and data maps • Project focussed teams created • More agile decision making process • Change status visible at all times |
Related Quality Management under MTP 4.0 articles:
ISPE Pharma 4.0TM – is a trademark registered with the European Union Intellectual Property Office and owned by the International Society for Pharmaceutical Engineering, Inc.
