A leading pharmaceutical manufacturer in Vietnam requested SeerPharma to conduct an on-site review of a manufacturing line, in preparation for an impending European Union (EU) Good Manufacturing Practice (GMP) inspection.
Infographic: Performance Snapshot for 2022
INFOGRAPHIC: A snapshot of how SeerPharma supported its customers with Quality and GMP Best-Practices in 2022.
Personnel behaviour plays a significant role in the manufacture of sterile products. Aseptic processing occurs under a Laminar Air Flow, which is classified as a Grade A or ISO 4.8 environment. Disturbance of air in the Laminar Air Flow can affect the quality of the final product.
Infographic: Performance Snapshot for 2021
INFOGRAPHIC: A snapshot of how SeerPharma supported its customers with Quality and GMP Best-Practices in 2021.
Human Error is the propensity for certain common mistakes by people; the making of an error as a natural result of being human. Human errors cannot be stopped by wishing they would not happen. The extent of knowledge, training and level of skill has little to do with the mistakes we make. However, most human Error is PREVENTABLE. Our systems and...
Infographic: Performance Snapshot for 2020
INFOGRAPHIC: A snapshot of how SeerPharma supported its customers with Quality and GMP Best-Practices in 2020.
The Pharmaceutical and Medical Device industries rely on a fragmented and complex supply chain to get product in to market. Your organisation or your suppliers could be conducting a single step or multiple steps of manufacture, and as such must adhere to the principles of GMP that apply to your operation and the market in which your product is...
Virtual QA and GMP Consulting
For over 30 years SeerPharma has aided Pharmaceutical and Medical Device companies on matters of Quality Assurance and GMP compliance.
The current COVID-19 pandemic is seeing day to day quality related business operations challenged by attempting to deal with multiple personnel across different departments remotely. The need for immediate and...
Infographic: QA & GMP Compliance Performance Snapshot for 2019
INFOGRAPHIC: A snapshot of how SeerPharma supported its clients in 2019 on matters of Quality Assurance and GMP compliance.
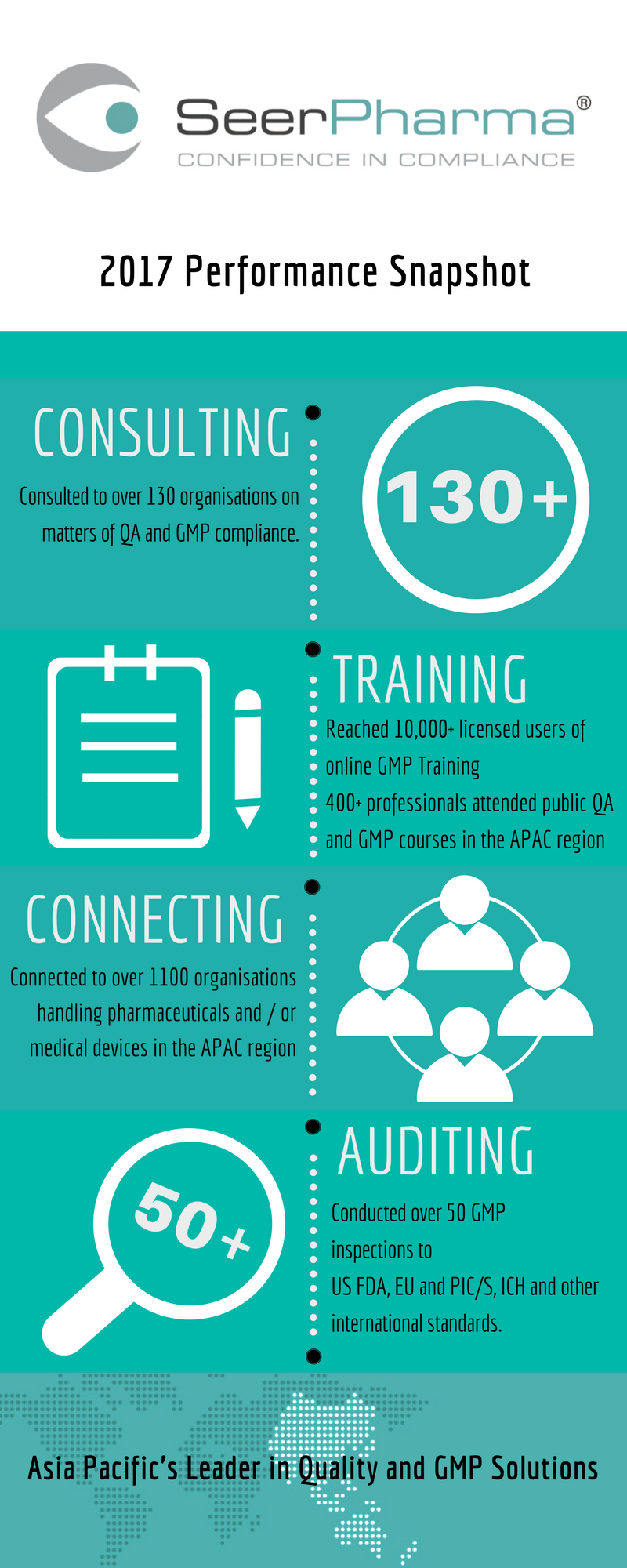
Infographic: QA & GMP Compliance Performance Snapshot for 2017
INFOGRAPHIC: A snapshot of how SeerPharma has supported its clients in 2017 on matters of QA and GMP compliance.








.png)