As medical devices become more sophisticated, adaptable to technological advances and accessible to the public, it is critical that companies ensure they have developed a robust program of usability engineering, to minimize the risk of incorrect usage, and create devices that are easy to use, intuitive and safe.
MasterControl Webinar: Medical Device Quality & Manufacturing Trends
SeerPharma are strategic partners with market leading software solution provider – MasterControl. Our partnership, over the last 7 years, has seen us deploy this solution to digitise Quality and Manufacturing workflows to over 75 companies.
MasterControl is running a webinar on current industry trends facing Quality and Manufacturing professionals...
2024 Medical Device Industry Trends
SeerPharma has over 30 years of experience, supporting diagnostic manufacturers and laboratories on matters related to Quality and GxP compliance. In that time , we’ve seen the emergence and importance of Point of Care Diagnostics (POCD). Following this trend, we’re delighted to be sponsoring an upcoming Biosymposium by the Biomelbourne Network...
In 2014, the FDA issued a guidance document for the Management of Cybersecurity in Medical Devices – Content of Premarket Submissions. Since then, technologies and the interconnectivity of medical devices have evolved rapidly. In response, the FDA released a new draft guidance in April 2022, for comment purposes only - Cybersecurity in Medical...
SeerPharma to Sponsor 8th Annual BioMelbourne Device + Diagnostics Lab
SeerPharma is excited to be sponsoring BioMelbourne Network’s 8th Annual Device + Diagnostics Lab, to be held on Thursday the 5th of March.
CSV Assistance for Pharmaceutical and Medical Device Companies
Several pharmaceutical and medical device manufacturers in the Asia-Pacific region have approached and engaged SeerPharma for assistance on matters related to Computer System Validation (CSV). Typical requests have seen SeerPharma address computer systems validation approaches to meet the regulatory requirements of Annex 11 of the PIC/S Guide to...
A major sponsor and distributor of medical devices in Australia engaged SeerPharma to conduct a gap assessment of its Quality Management System (QMS) and Operations against ISO 13485:2016. The client's current operations are licensed to ISO 9001:2015 and required assistance from SeerPharma to understand what would be required to upgrade their QMS...
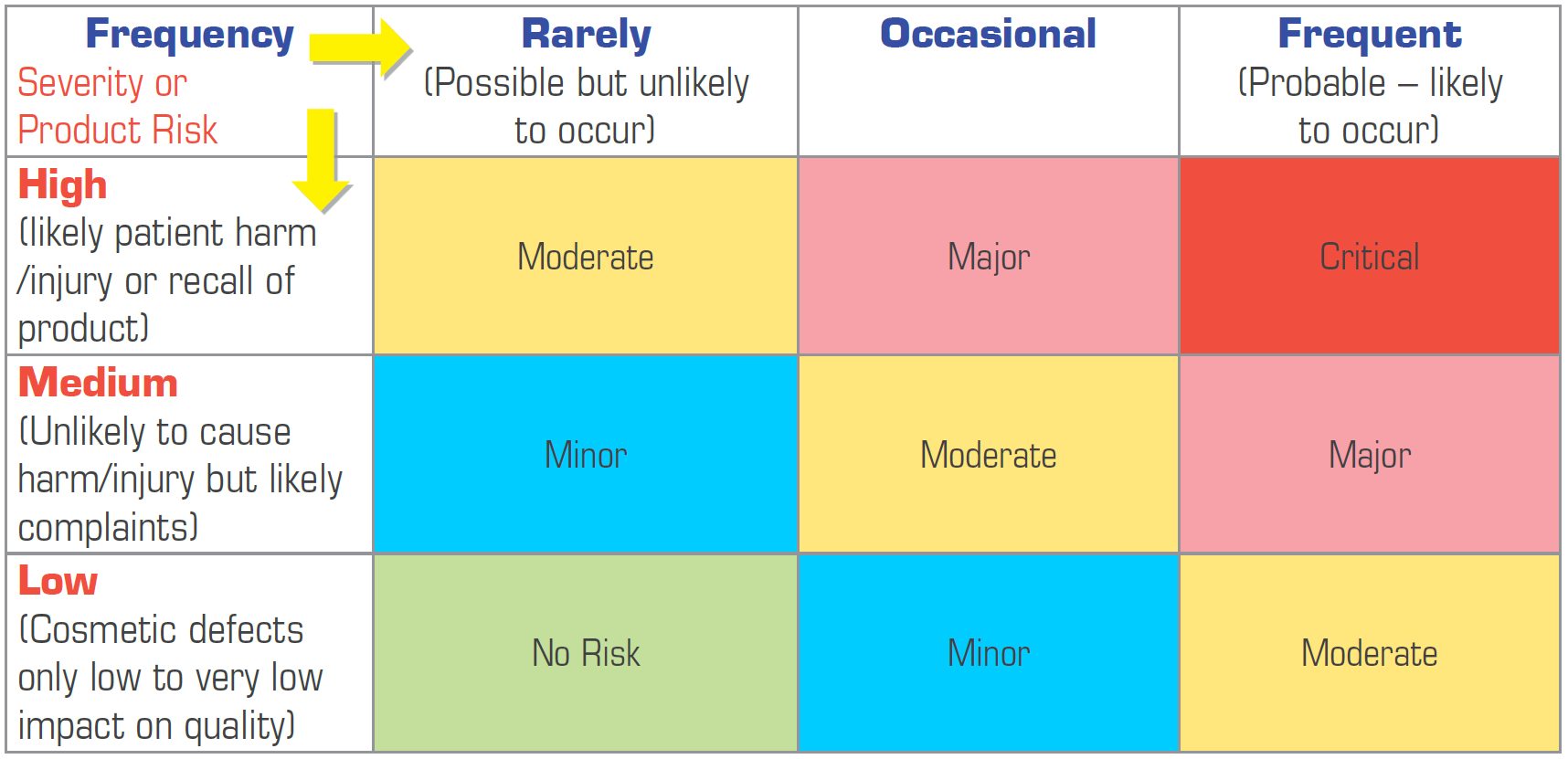
Application of ISO 14971 Risk Management to New Medical Devices
The stages required for applying ISO 14971 principles to risk management for medical devices can be typically broken into 6 steps: