INFOGRAPHIC: A snapshot of how SeerPharma supported its customers with Quality and GMP Best-Practices in 2025 by providing QA & GMP Consulting, Auditing & Training services as well as MasterControl solutions "Qx" electronic Quality Management Systems (eQMS) & "Mx" Manufacturing Execution Systems (MES) with electronic Batch Records (eBR).
Infographic: Performance Snapshot for 2024
INFOGRAPHIC: A snapshot of how SeerPharma supported its customers with Quality and GMP Best-Practices in 2024 by providing QA & GMP Consulting, Auditing & Training services as well as MasterControl solutions "Qx" electronic Quality Management Systems (eQMS) & "Mx" Manufacturing Execution Systems (MES) with electronic Batch Records (eBR).
QA / GMP Support for Pharmaceutical and Medical Device Companies
SeerPharma consults on 100 to 150 projects every year. Many of these engagements with pharmaceutical and medical device companies involve providing ongoing support for business needs related to Quality Assurance (QA) and Good Manufacturing Practice (GMP) compliance. Our clients continue to rely on our expertise for regular remote and on-site...
Infographic: Performance Snapshot for 2023
INFOGRAPHIC: A snapshot of how SeerPharma supported its clients with Quality and GMP Best-Practices in 2023 through QA & GMP Consulting, Auditing, Training and MasterControl "Qx" electronic Quality Management Systems (eQMS) & "Mx" Manufacturing Execution Systems (MES) with electronic Batch Records (eBR).
Infographic: Performance Snapshot for 2022
INFOGRAPHIC: A snapshot of how SeerPharma supported its customers with Quality and GMP Best-Practices in 2022.
Infographic: Performance Snapshot for 2021
INFOGRAPHIC: A snapshot of how SeerPharma supported its customers with Quality and GMP Best-Practices in 2021.
Infographic: Performance Snapshot for 2020
INFOGRAPHIC: A snapshot of how SeerPharma supported its customers with Quality and GMP Best-Practices in 2020.
Virtual QA and GMP Consulting
For over 30 years SeerPharma has aided Pharmaceutical and Medical Device companies on matters of Quality Assurance and GMP compliance.
The current COVID-19 pandemic is seeing day to day quality related business operations challenged by attempting to deal with multiple personnel across different departments remotely. The need for immediate and...
Infographic: QA & GMP Compliance Performance Snapshot for 2019
INFOGRAPHIC: A snapshot of how SeerPharma supported its clients in 2019 on matters of Quality Assurance and GMP compliance.
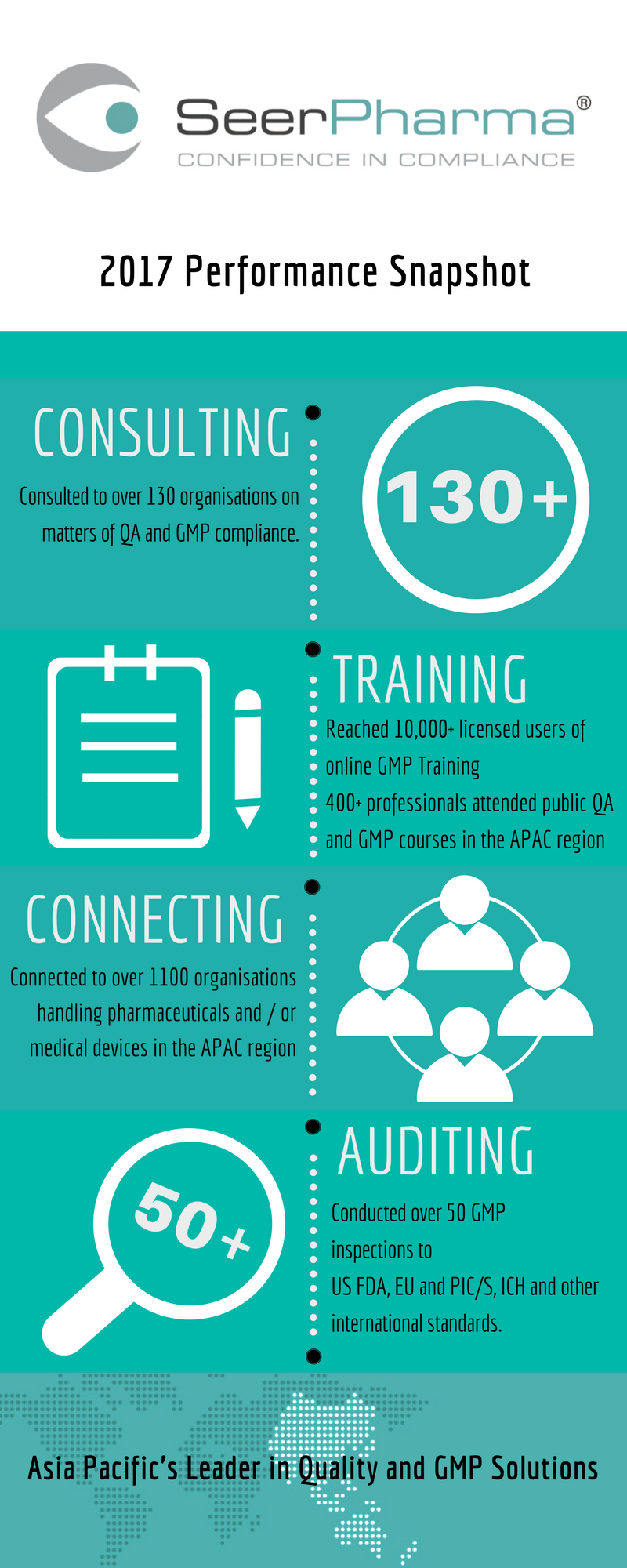
Infographic: QA & GMP Compliance Performance Snapshot for 2017
INFOGRAPHIC: A snapshot of how SeerPharma has supported its clients in 2017 on matters of QA and GMP compliance.









.png)