INFOGRAPHIC: A snapshot of how SeerPharma supported its customers with Quality and GMP Best-Practices in 2025 by providing QA & GMP Consulting, Auditing & Training services as well as MasterControl solutions "Qx" electronic Quality Management Systems (eQMS) & "Mx" Manufacturing Execution Systems (MES) with electronic Batch Records (eBR).
Infographic: Performance Snapshot for 2024
INFOGRAPHIC: A snapshot of how SeerPharma supported its customers with Quality and GMP Best-Practices in 2024 by providing QA & GMP Consulting, Auditing & Training services as well as MasterControl solutions "Qx" electronic Quality Management Systems (eQMS) & "Mx" Manufacturing Execution Systems (MES) with electronic Batch Records (eBR).
The inclusion of medical devices onto the Therapeutic Goods Administration's (TGA’s) Australian Register of Therapeutic Goods (ARTG) requires manufacturers to demonstrate that their device meets specific safety and performance characteristics and has been designed and manufactured in accordance with the Essential Principles.
Infographic: Performance Snapshot for 2023
INFOGRAPHIC: A snapshot of how SeerPharma supported its clients with Quality and GMP Best-Practices in 2023 through QA & GMP Consulting, Auditing, Training and MasterControl "Qx" electronic Quality Management Systems (eQMS) & "Mx" Manufacturing Execution Systems (MES) with electronic Batch Records (eBR).
Infographic: Performance Snapshot for 2022
INFOGRAPHIC: A snapshot of how SeerPharma supported its customers with Quality and GMP Best-Practices in 2022.
Infographic: Performance Snapshot for 2021
INFOGRAPHIC: A snapshot of how SeerPharma supported its customers with Quality and GMP Best-Practices in 2021.
Infographic: Performance Snapshot for 2020
INFOGRAPHIC: A snapshot of how SeerPharma supported its customers with Quality and GMP Best-Practices in 2020.
Infographic: QA & GMP Compliance Performance Snapshot for 2019
INFOGRAPHIC: A snapshot of how SeerPharma supported its clients in 2019 on matters of Quality Assurance and GMP compliance.
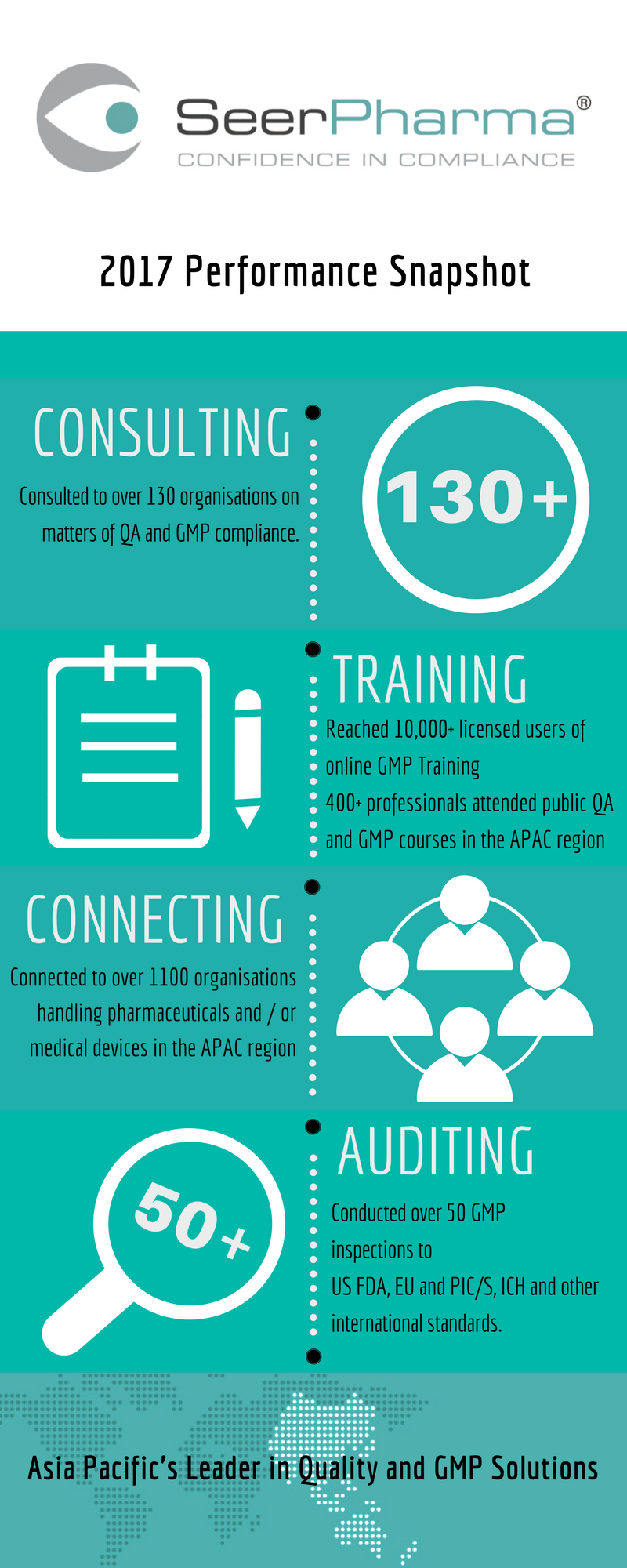
Infographic: QA & GMP Compliance Performance Snapshot for 2017
INFOGRAPHIC: A snapshot of how SeerPharma has supported its clients in 2017 on matters of QA and GMP compliance.
INFOGRAPHIC: A snapshot of how SeerPharma has supported its clients in 2016 on matters of QA and GMP compliance.








.png)