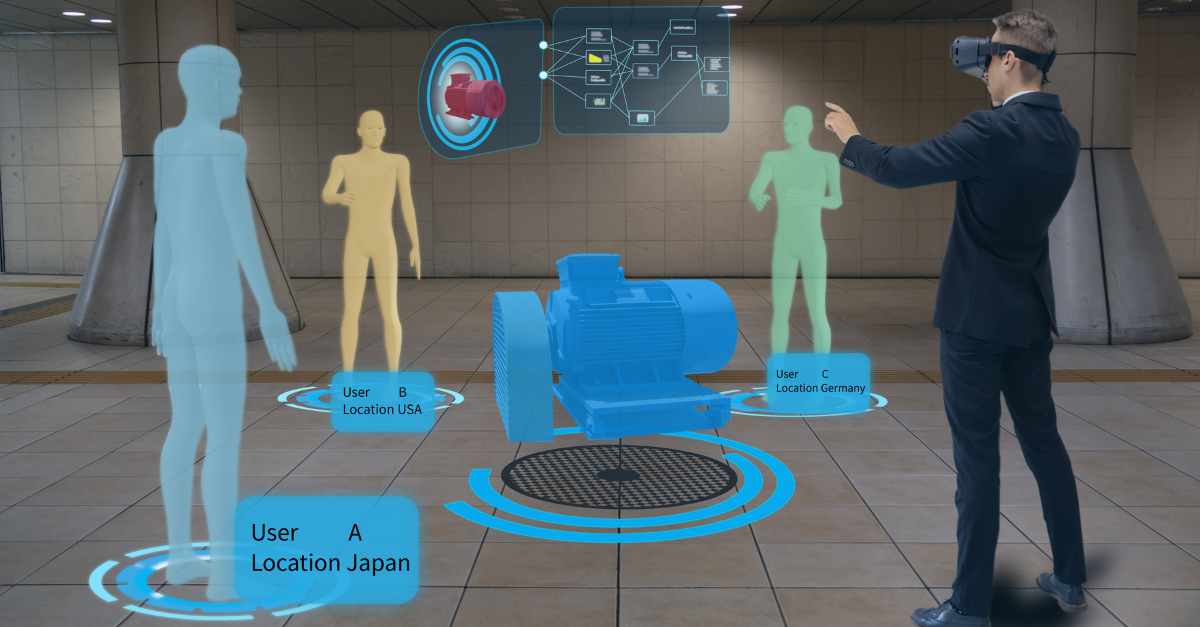
Process Validation: A Universal Concept in the Regulatory Landscape
Across the pharmaceutical, biopharmaceutical and medical device industries, process validation has historically been structured around the trilogy:
- Process Design: Develop and understand the process based on science, risk, and data (Stage 1)
- Process Qualification: Confirm the...