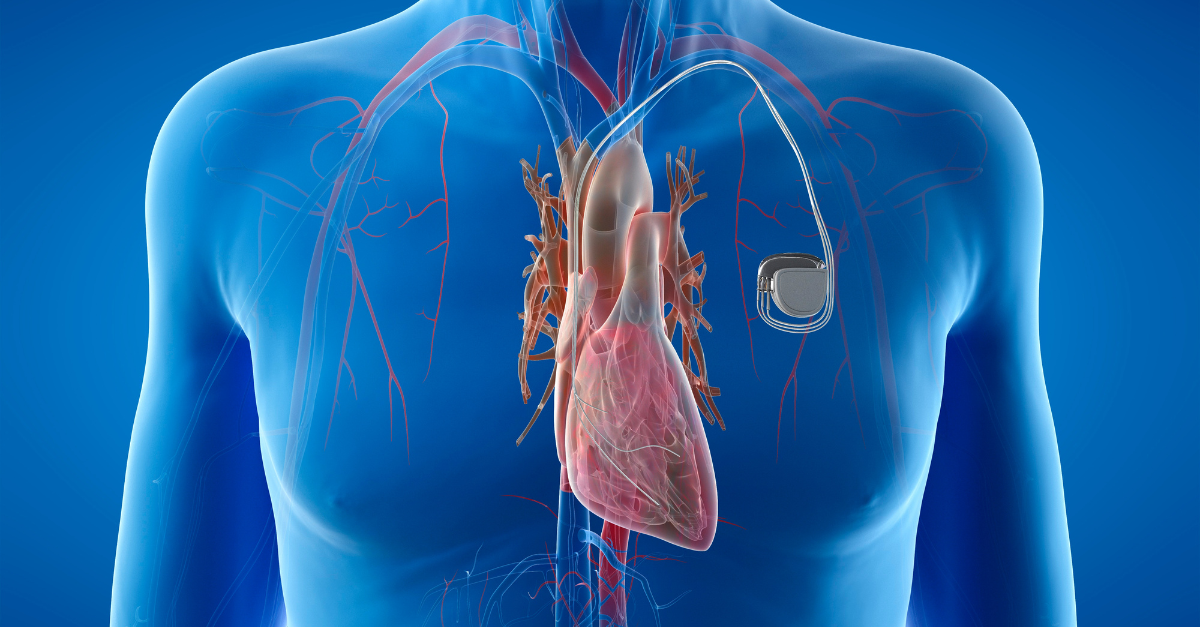
Entering the medical device industry is an exciting venture—but it comes with a maze of regulatory requirements that can feel overwhelming, especially for startups and new manufacturers. One acronym you’ll encounter early and often is MDSAP: the Medical Device Single Audit Program.
Conducting a Mock FDA Audit of Medical Device Manufacturer (Singapore)
A multinational manufacturer of cardiovascular devices recently engaged SeerPharma to conduct a comprehensive mock US FDA audit of their operations in Singapore. The audit was conducted against US FDA 21 CFR Part 820 – Quality System Regulation (21 CFR 820). SeerPharma reviewed relevant documentation remotely and then spent three days on-site...
SeerPharma has been engaged by multiple companies to conduct GMP audits in the Asia-Pacific region on behalf of Qualified Persons (QP) in Europe.
Industry 4.0, or the Fourth Industrial Revolution is a term used to describe the use of smart technology in manufacturing and industrial processes.
ISPE (the International Society for Pharmaceutical Engineering) has trademarked the term ‘ISPE Pharma 4.0’ to cover Industry 4.0 in the life sciences sector.
As the Industry 4.0 concept is equally...
The Pharmaceutical and Medical Device industries rely on a fragmented and complex supply chain to get product in to market. Your organisation or your suppliers could be conducting a single step or multiple steps of manufacture, and as such must adhere to the principles of GMP that apply to your operation and the market in which your product is...
INFOGRAPHIC: A snapshot of how SeerPharma has supported its clients in 2016 on matters of QA and GMP compliance.
EU GMP and FDA GMP Audit
SeerPharma was asked to conduct a mock GMP audit of a Korean Pharmaceutical company that produces non-sterile solid dosage forms and finished products.
GMP Supplier Audits in Europe and Asia
A number of firms have requested our expertise to provide technical and compliance audits of contract manufacturers that they currently use in Europe and Asia.
Implementing FDA, EMA and PIC/S GMP
SeerPharma (Singapore) was engaged by a company in Singapore to help implement cGMP for a new clinical trial packaging facility and obtain international certification of conformance to FDA, EMA and PIC/S GMP.
A US-based biopharmaceutical firm has approached SeerPharma to perform audits of their critical service providers. The company is about to undertake a phase I clinical trial in Australia for one of its therapeutic molecules.