This article was originally published by SeerPharma's business partner MasterControl and is republished in-part here with their permission.
Human skin is home to many colonies of microorganisms referred to as skin microbiota or skin flora. In addition to these resident microorganisms, human skin may become contaminated by foreign organisms that are transferred from the environment or other parts of the body (i.e. respiratory exhalation, different skin areas).
I am currently the Quality Manager of BJP Laboratories Pty Ltd, located in Yatala, Queensland. BJP is a contract manufacturer of TGA-listed complementary medicines and dietary supplements. Our facility is also licensed with the APVMA and registered with the US-FDA. Before this role, I worked as Quality Assurance Manager of Mavlab Animal Health...
Meet 2022 SeerPharma Scholarship Recipient: Tanya Goodwin
I grew up in Far North Queensland, surrounded by the Daintree rainforest and the Great Barrier Reef Marine Park. This sparked my passion for investigating tropical fruits and the health benefits of traditional medicines.
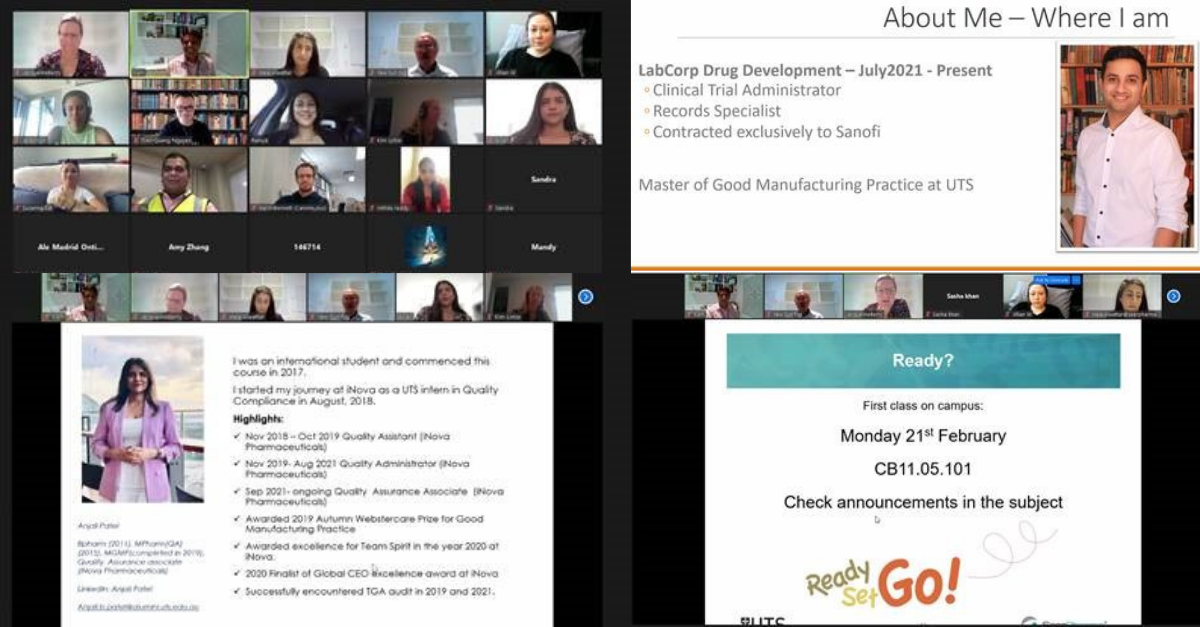
It's been another challenging year in the higher education sector, so SeerPharma is particularly excited to welcome the 2022 cohort of postgraduate students studying Good Manufacturing Practice (GMP) at University of Technology Sydney (UTS). This cohort is a global blend of graduates and industry professionals.
SeerPharma Offer MasterControl Implementation Services in Nth America
SeerPharma has been a strategic Partner for MasterControl in the Asia-Pacific region for over 5 years. In that time, we've secured business and implemented this solution for over 60 organisations in the Pharmaceutical and Medical device sector, helping them automate business-critical Quality and Manufacturing workflows.
Free Webinar: Automating Document, Trial and Quality Management
Medical Technology, Biotechnology & Pharmaceutical Industry 4.0 Forum
The ISPE Australasian Affiliate and PDA Australia Chapter, with the support of MTPConnect, will be holding a free online forum on Industry 4.0, or the fourth industrial revolution, and its impact on the Medical Technology, Biotechnology and Pharmaceutical (MTP) industries.
Infographic: Performance Snapshot for 2021
INFOGRAPHIC: A snapshot of how SeerPharma supported its customers with Quality and GMP Best-Practices in 2021.
In Hong Kong, the Authorised Person (AP) must ensure that all legislative obligations are fully satisfied before any pharmaceutical product is released for sale or supply.