Let’s just say what we’re all thinking: 2020 was rough. It threw every element of our lives into disarray. We suddenly had to become experts at navigating a new way of working, schooling and socialising. It was disruptive and it was challenging, but it was also transformative. And while the past 12 months might have felt like 12 years, it’s time...
MasterControl Manufacturing Software Solutions
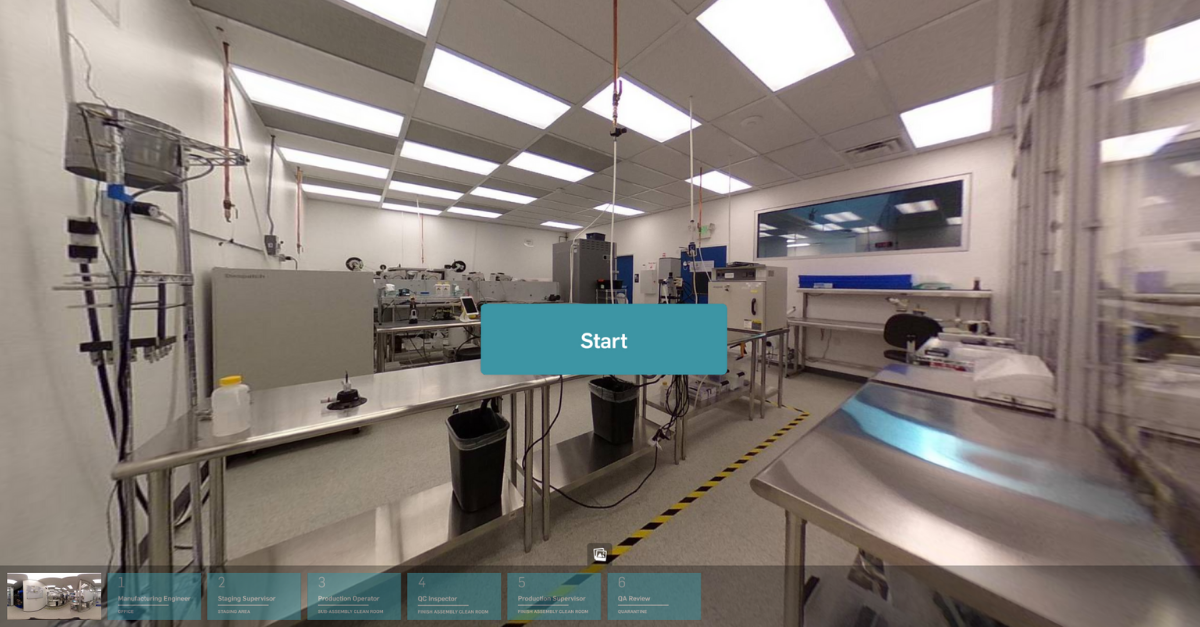
MasterControl delivers the next level of manufacturing excellence by bridging the gap between performance and quality with digital production records that finally let you go 100% paperless.
MasterControl Manufacturing Excellence (Mx)
Shaping the Next Normal for Quality and Compliance: 2021 QM Trends
Let’s just say what we’re all thinking: 2020 was rough. It threw every element of our lives into disarray. We suddenly had to become experts at navigating a new way of working, schooling and socialising. It was disruptive and it was challenging, but it was also transformative. And although the past 12 months might have felt like 12 years, it’s...
iNova Pharmaceuticals Engage SeerPharma to Support CSV Operating Model
iNova Pharmaceuticals (“iNova”) is an international organisation that develops, markets and sells a wide-range of prescription medicines and non-prescription consumer health products to over 20 countries across Asia, Australia and New Zealand, and Africa. Employing over 500 industry professionals over 3 continents, iNova’s vision is to deliver...
Adapting to Pharma’s Next Normal: 2021 Pharmaceutical Trends
Let’s just say what we’re all thinking: 2020 was rough. It threw every element of our lives into disarray. We suddenly had to become experts at navigating a new way of working, schooling and socialising. It was disruptive and it was challenging, but it was also transformative. And although the past 12 months might have felt like 12 years, it’s...
Infographic: Performance Snapshot for 2020
INFOGRAPHIC: A snapshot of how SeerPharma supported its customers with Quality and GMP Best-Practices in 2020.
SeerPharma Wins Masters of Excellence Partner Award from MasterControl
MasterControl Recognizes Partner Achievements
The first annual Masters of Excellence Partner Award highlights success.
SALT LAKE CITY, Utah: November 24, 2020-- MasterControl, a provider of software solutions that enable life sciences and other regulated companies to deliver life-changing products to more people sooner, announced its first...
Theoretical vs Tactical Good Manufacturing Practices Lunch and Learn
15 December 2020 | 12:00 p.m. AEDT
It’s vital to follow the latest good manufacturing practices. But what are the most efficient and effective ways to put GMP theories into action on your shop floor? Join MasterControl’s Ciaran O'Keeffe and Luana Carone as they welcome David Spaulding and Rohan Bhatia from SeerPharma for this free lunch and...
MasterControl Launch Manufacturing Excellence for the APAC Region
With cloud infrastructure now in place in Australia, Singapore, Japan, China and India through Amazon Web Services (AWS), MasterControl have launched their latest Manufacturing Software Solution – Manufacturing Excellence (Mx) to the Asia-Pacific market.
Overview of ISO 13485 – Medical Device QMS Requirements
This article was originally published by SeerPharma's business partner MasterControl and is republished here with their permission.
Some medical devices are as complex as a remote, personalized heart failure sensor. Others are as simple as a tongue depressor. But all medical devices have one thing in common: they benefit immensely from being...






.png)


